TR-14035╬▒4╬▓7 and ╬▒4╬▓1 integrins antagonist CAS# 232271-19-1 |
- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
- BMS-345541
Catalog No.:BCC1423
CAS No.:547757-23-3
- Bay 65-1942 free base
Catalog No.:BCC1408
CAS No.:600734-02-9
- Bay 65-1942 HCl salt
Catalog No.:BCC1409
CAS No.:600734-06-3
- Bay 65-1942 R form
Catalog No.:BCC1410
CAS No.:758683-21-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 232271-19-1 | SDF | Download SDF |
| PubChem ID | 9912743 | Appearance | Powder |
| Formula | C24H21Cl2NO5 | M.Wt | 474.33 |
| Type of Compound | N/A | Storage | Desiccate at -20┬░C |
| Solubility | DMSO : Ōēź 41 mg/mL (86.44 mM) *"Ōēź" means soluble, but saturation unknown. | ||
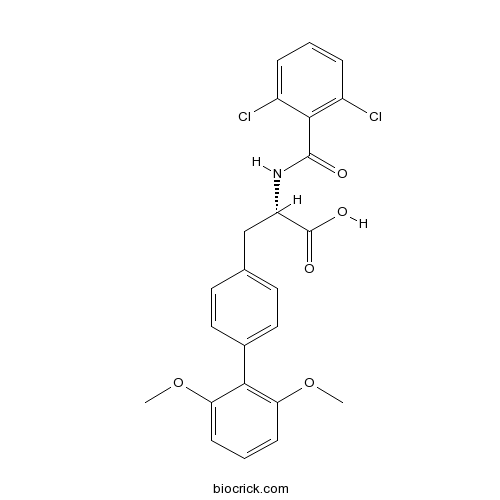
| Chemical Name | (2S)-2-[(2,6-dichlorobenzoyl)amino]-3-[4-(2,6-dimethoxyphenyl)phenyl]propanoic acid | ||
| SMILES | COC1=C(C(=CC=C1)OC)C2=CC=C(C=C2)CC(C(=O)O)NC(=O)C3=C(C=CC=C3Cl)Cl | ||
| Standard InChIKey | DRSJLVGDSNWQBI-SFHVURJKSA-N | ||
| Standard InChI | InChI=1S/C24H21Cl2NO5/c1-31-19-7-4-8-20(32-2)21(19)15-11-9-14(10-12-15)13-18(24(29)30)27-23(28)22-16(25)5-3-6-17(22)26/h3-12,18H,13H2,1-2H3,(H,27,28)(H,29,30)/t18-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 Ōäā and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20Ōäā for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20Ōäā. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | TR-14035 is a a dual alpha4beta7(IC50=7 nM)/alpha4beta1 (IC50=87 nM) integrin antagonist .
IC50 Value: alpha(4)beta(7)/alpha(4)beta(1)=7/87 nM [1]
Target: integrin
TR14035 blocked the binding of human alpha(4)beta(7) to an (125)I-MAdCAM-Ig fusion protein with IC(50) values of 0.75 nM. TR14035 blocked binding of human alpha(4)beta(7)-expressing RPMI-8866 cells or murine mesenteric lymph node lymphocytes to MAdCAM-Ig with IC(50) values of 0.1 microM [2].
TR14035 blocked adhesion to HEVs [ED(50) of 0.01-0.1 mpk i.v.].
TR-14035 was taken up by rat and human hepatocytes by an apparently single saturable mechanism with K(m) of 6.7 and 2.1 microM, respectively, and taurocholate and digoxin reduced this uptake [3]. References: | |||||

TR-14035 Dilution Calculator

TR-14035 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1082 mL | 10.5412 mL | 21.0824 mL | 42.1647 mL | 52.7059 mL |
| 5 mM | 0.4216 mL | 2.1082 mL | 4.2165 mL | 8.4329 mL | 10.5412 mL |
| 10 mM | 0.2108 mL | 1.0541 mL | 2.1082 mL | 4.2165 mL | 5.2706 mL |
| 50 mM | 0.0422 mL | 0.2108 mL | 0.4216 mL | 0.8433 mL | 1.0541 mL |
| 100 mM | 0.0211 mL | 0.1054 mL | 0.2108 mL | 0.4216 mL | 0.5271 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
TR-14035 is a dual antagonist of ╬▒4╬▓7 and ╬▒4╬▓1 integrins with IC50 value of 7nM and 87nM, respectively [1].
The inhibitors of ╬▒4╬▓7 and ╬▒4╬▓1 integrins are developed for treatment of inflammation and autoimmune diseases such as asthma and rheumatoid arthritis. TR-14035 is the first orally bioavailable chemical entity. It is screened out by the RPMI assay and the Jurkat cell adhesion assay. The IC50 values of it in Jurkat CS1 assay for ╬▒4╬▓7 and ╬▒4╬▓1 are 7nM and 87nM, respectively. Furthermore, the oral bioavailability of TR-14035 is limited due to the combination of dissolution rate, hepatic first-pass metabolism and biliary excretion. Besides that, TR-14035 displays species differences in the pharmacokinetics and disposition between the rat and dog [1, 2].
References:
[1] Sircar I, Gudmundsson K S, Martin R, et al. Synthesis and SAR of N-Benzoyl-l-Biphenylalanine Derivatives: Discovery of TR-14035, A Dual ╬▒4╬▓7/╬▒4╬▓1 Integrin Antagonist. Bioorganic & medicinal chemistry, 2002, 10(6): 2051-2066.
[2] Tsuda-Tsukimoto M, Ogasawara Y, Kume T. Pharmacokinetics and metabolism of TR-14035, a novel antagonist of ╬▒4╬▓1/╬▒4╬▓7 integrin mediated cell adhesion, in rat and dog. Xenobiotica, 2005, 35(4): 373-389.
- 23-Hydroxymangiferonic acid
Catalog No.:BCN4668
CAS No.:232266-08-9
- Methoxydienone
Catalog No.:BCC9030
CAS No.:2322-77-2
- Doxorubicin
Catalog No.:BCC2082
CAS No.:23214-92-8
- Ifenprodil Tartrate
Catalog No.:BCC4589
CAS No.:23210-58-4
- Ifenprodil hemitartrate
Catalog No.:BCC6688
CAS No.:23210-56-2
- Matairesinoside
Catalog No.:BCN7583
CAS No.:23202-85-9
- (1R,2S)-2-Amino-1,2-diphenylethanol
Catalog No.:BCC8382
CAS No.:23190-16-1
- Columbianetin acetate
Catalog No.:BCN2652
CAS No.:23180-65-6
- Paeoniflorin
Catalog No.:BCN6301
CAS No.:23180-57-6
- Simiarenone
Catalog No.:BCN5082
CAS No.:2318-78-7
- Senkirkine
Catalog No.:BCN2136
CAS No.:2318-18-5
- Songoramine
Catalog No.:BCN6474
CAS No.:23179-78-4
- Ritodrine HCl
Catalog No.:BCC4337
CAS No.:23239-51-2
- 5,7-Diacetoxy-8-methoxyflavone
Catalog No.:BCN5083
CAS No.:23246-80-2
- Riddelline
Catalog No.:BCN2133
CAS No.:23246-96-0
- Dimaprit dihydrochloride
Catalog No.:BCC6672
CAS No.:23256-33-9
- Guanabenz Acetate
Catalog No.:BCC4327
CAS No.:23256-50-0
- 4'-O-Methylvitexin
Catalog No.:BCN2642
CAS No.:2326-34-3
- Bay 36-7620
Catalog No.:BCC5915
CAS No.:232605-26-4
- VAL-083
Catalog No.:BCC2024
CAS No.:23261-20-3
- m-NH2-Tyr-OH.2HCl
Catalog No.:BCC3340
CAS No.:23279-22-3
- Probucol
Catalog No.:BCC4833
CAS No.:23288-49-5
- Delta 7-avenasterol
Catalog No.:BCN3212
CAS No.:23290-26-8
- Emodin-8-beta-D-glucoside
Catalog No.:BCN6329
CAS No.:23313-21-5
Pharmacokinetics and metabolism of TR-14035, a novel antagonist of a4ss1/a4ss7 integrin mediated cell adhesion, in rat and dog.[Pubmed:16019958]
Xenobiotica. 2005 Apr;35(4):373-89.
The pharmacokinetics and disposition of N-(2,6-dichlorobenzoyl)-4-(2,6-dimethoxyphenyl)-L-phenylalanine (TR-14035), a novel a4ss1/a4ss7 antagonist, were investigated in the rat and dog. Results indicate extensive clearance of TR-14035 and low oral bioavailability, 17% and 13% in the rat and dog, respectively, at an oral dose of 10 mg/kg. At least 63% of the oral dose was absorbed from the gastrointestinal tract in the rat, and about one-third of the intravenous dose was excreted into bile as unchanged drug in the rat and dog. These data indicate that the oral bioavailability of TR-14035 was limited due to significant first-pass metabolism and biliary excretion in the liver. A species-dependent difference in metabolism was observed. The principal metabolite, O-desmethyl TR-14035, observed in rat, dog and probably human, was further conjugated with sulfate in the rat, but never in dog and human, based on in vitro metabolism and in vivo metabolite profile studies. Urinary excretion was a minor elimination route, but an interesting species difference was recognized. TR-14035 was reabsorbed from the rat renal proximal tubules, and by contrast, secreted into the tubules in the dog, probably via active transport systems.
Synthesis and SAR of N-benzoyl-L-biphenylalanine derivatives: discovery of TR-14035, a dual alpha(4)beta(7)/alpha(4)beta(1) integrin antagonist.[Pubmed:11937364]
Bioorg Med Chem. 2002 Jun;10(6):2051-66.
alpha(4)beta(1) and alpha(4)beta(7) integrins are key regulators of physiologic and pathologic responses in inflammation and autoimmune disease. The effectiveness of anti-integrin antibodies to attenuate a number of inflammatory/immune conditions provides a strong rationale to target integrins for drug development. Important advances have been made in identifying potent and selective candidates, peptides and peptidomimetics, for further development. Herein, we report the discovery of a series of novel N-benzoyl-L-biphenylalanine derivatives that are potent inhibitors of alpha4 integrins. The potency of the initial lead compound (1: IC(50) alpha(4)beta(7)/alpha(4)beta(1)=5/33 microM) was optimized via sequential manipulation of substituents to generate low nM, orally bioavailable dual alpha(4)beta(1)/alpha(4)beta(7) antagonists. The SAR also led to the identification of several subnanomolar antagonists (134, 142, and 143). Compound 81 (TR-14035; IC(50) alpha(4)beta(7)/alpha(4)beta(1)=7/87 nM) has completed Phase I studies in Europe. The synthesis, SAR and biological evaluation of these compounds are described.
Role of human liver cytochrome P450 2C9 in the metabolism of a novel alpha4beta1/alpha4beta7 dual antagonist, TR-14035.[Pubmed:15855725]
Drug Metab Pharmacokinet. 2005 Apr;20(2):127-34.
The metabolism of a novel dual antagonist for alpha4beta1/alpha4beta7 integrin, TR-14035, and the role of polymorphic enzyme responsible for this metabolism were investigated. Human liver microsomes catalyzed the NADPH-dependent metabolism of TR-14035 to a primary metabolite, O-desmethyl TR-14035. This formation was completely blocked by both sulfaphenazole, a selective CYP2C9 inhibitor, and CYP2C9 antibody, whereas potent inhibitors selective for other CYPs exhibited little effects. Of 12 recombinant CYPs examined, O-desmethyl metabolite was principally formed by CYP2C9. CYP1A1, an extrahepatic enzyme, also had this activity (about one-fourth of CYP2C9). Utilizing recombinant CYP2C9*1, K(m) and V(max)/K(m) values of 23.3 microM and 0.284 microL/min/pmol CYP2C9, respectively, were obtained for the O-desmethyl formation, which were quite similar to those in CYP2C9*2 enzyme. In contrast, V(max)/K(m) value in recombinant CYP2C9*3 was approximately one-sixth of CYP2C9*1 and *2. In agreement, kinetics studies using human liver microsomes with CYP2C9*1/*1, *2/*2 and *3/*3 genotypes revealed that the V(max)/K(m) value in *2/*2 microsomes was comparable to that in wild type microsomes, in contrast, that in *3/*3 microsomes was reduced. These results demonstrate CYP2C9 is a primary enzyme mediating the O-desmethylation of TR-14035 in human liver. In homozygotes of CYP2C9*3, the metabolic clearance of TR-14035 should be decreased compared with homozygotes of CYP2C9*1 or 2.
Characterization of hepatobiliary transport systems of a novel alpha4beta1/alpha4beta7 dual antagonist, TR-14035.[Pubmed:16969695]
Pharm Res. 2006 Nov;23(11):2646-56.
PURPOSE: Our previous pharmacokinetic studies have demonstrated that TR-14035, a novel dual antagonist for alpha4beta1/alpha4beta7 integrin, selectively and strongly accumulated in the liver and was mainly excreted in bile as an unchanged drug. In the present study, we investigated the hepatobiliary transport system in detail. MATERIALS AND METHODS: Uptake by hepatocytes and organic anion transporting polypeptide (OATP)-expressing Xenopus laevis oocytes or Flp-In-293 cells was performed in vitro. Biliary excretion was investigated in mdr1a/b-knockout mice, Bcrp-knockout mice and Mrp2-defective Eisai hyperbilirubinemic rats (EHBRs). RESULTS: TR-14035 was taken up by rat and human hepatocytes by an apparently single saturable mechanism with K(m) of 6.7 and 2.1 microM, respectively, and taurocholate and digoxin reduced this uptake. OATP1B1/OATP-C and OATP1B3/OATP8 expressed in oocytes mediated the TR-14035 uptake with K(m) of 7.5 and 5.3 microM, respectively. OATP1B1*15, a genetic variant of OATP1B1, exhibited a decreased transport of TR-14035 compared with OATP1B1*1a. Biliary excretion and total body clearance of unchanged TR-14035 in EHBRs were significantly lower than those in normal rats, while there was no difference in the clearances between wild and mdr1a/b- or Bcrp-knockout mice. CONCLUSION: These results indicate that OATP1B1 and OATP1B3 are at least partly responsible for the accumulation of TR-14035 into hepatocytes, and Mrp2 principally mediates the biliary excretion of TR-14035. Furthermore, genetic polymorphisms of OATP1B1 may cause an interindividual variability in the pharmacokinetics of TR-14035.


