ProbucolAntioxidant, anti-inflammatory and hypocholesterolemic agent CAS# 23288-49-5 |
- Laminin (925-933)
Catalog No.:BCC1015
CAS No.:110590-60-8
- Epidermal Growth Factor Receptor Peptide (985-996)
Catalog No.:BCC1014
CAS No.:96249-43-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 23288-49-5 | SDF | Download SDF |
| PubChem ID | 4912 | Appearance | Powder |
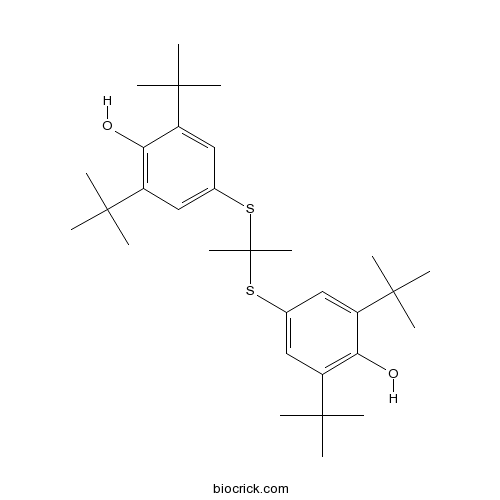
| Formula | C31H48O2S2 | M.Wt | 516.84 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (193.48 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 2,6-ditert-butyl-4-[2-(3,5-ditert-butyl-4-hydroxyphenyl)sulfanylpropan-2-ylsulfanyl]phenol | ||
| SMILES | CC(C)(C)C1=CC(=CC(=C1O)C(C)(C)C)SC(C)(C)SC2=CC(=C(C(=C2)C(C)(C)C)O)C(C)(C)C | ||
| Standard InChIKey | FYPMFJGVHOHGLL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C31H48O2S2/c1-27(2,3)21-15-19(16-22(25(21)32)28(4,5)6)34-31(13,14)35-20-17-23(29(7,8)9)26(33)24(18-20)30(10,11)12/h15-18,32-33H,1-14H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Antioxidant, anti-inflammatory and hypocholesterolemic agent. Inhibits atherogenesis in genetically hypercholesterolemic rabbits (Watanabe) and attenuates ischemia/reperfusion-induced cardiomyocyte apoptosis. |

Probucol Dilution Calculator

Probucol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9348 mL | 9.6742 mL | 19.3483 mL | 38.6967 mL | 48.3709 mL |
| 5 mM | 0.387 mL | 1.9348 mL | 3.8697 mL | 7.7393 mL | 9.6742 mL |
| 10 mM | 0.1935 mL | 0.9674 mL | 1.9348 mL | 3.8697 mL | 4.8371 mL |
| 50 mM | 0.0387 mL | 0.1935 mL | 0.387 mL | 0.7739 mL | 0.9674 mL |
| 100 mM | 0.0193 mL | 0.0967 mL | 0.1935 mL | 0.387 mL | 0.4837 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Probucol
- m-NH2-Tyr-OH.2HCl
Catalog No.:BCC3340
CAS No.:23279-22-3
- VAL-083
Catalog No.:BCC2024
CAS No.:23261-20-3
- Bay 36-7620
Catalog No.:BCC5915
CAS No.:232605-26-4
- 4'-O-Methylvitexin
Catalog No.:BCN2642
CAS No.:2326-34-3
- Guanabenz Acetate
Catalog No.:BCC4327
CAS No.:23256-50-0
- Dimaprit dihydrochloride
Catalog No.:BCC6672
CAS No.:23256-33-9
- Riddelline
Catalog No.:BCN2133
CAS No.:23246-96-0
- 5,7-Diacetoxy-8-methoxyflavone
Catalog No.:BCN5083
CAS No.:23246-80-2
- Ritodrine HCl
Catalog No.:BCC4337
CAS No.:23239-51-2
- TR-14035
Catalog No.:BCC4266
CAS No.:232271-19-1
- 23-Hydroxymangiferonic acid
Catalog No.:BCN4668
CAS No.:232266-08-9
- Methoxydienone
Catalog No.:BCC9030
CAS No.:2322-77-2
- Delta 7-avenasterol
Catalog No.:BCN3212
CAS No.:23290-26-8
- Emodin-8-beta-D-glucoside
Catalog No.:BCN6329
CAS No.:23313-21-5
- Cephalexin monohydrate
Catalog No.:BCC4096
CAS No.:23325-78-2
- Nefopam HCl
Catalog No.:BCC4681
CAS No.:23327-57-3
- MK 0343
Catalog No.:BCC6170
CAS No.:233275-76-8
- Glycoborinine
Catalog No.:BCN7462
CAS No.:233279-39-5
- L-Ser(Bzl)-ol
Catalog No.:BCC2579
CAS No.:23356-96-9
- Vinleurosine
Catalog No.:BCN2608
CAS No.:23360-92-1
- (1S,2R)-2-Amino-1,2-diphenylethanol
Catalog No.:BCC8385
CAS No.:23364-44-5
- Theviridoside
Catalog No.:BCN5084
CAS No.:23407-76-3
- Phalaenopsine T
Catalog No.:BCN2014
CAS No.:23412-97-7
- Phalaenopsine La
Catalog No.:BCN2015
CAS No.:23412-99-9
Cell lipid metabolism modulators 2-bromopalmitate, D609, monensin, U18666A and probucol shift discoidal HDL formation to the smaller-sized particles: implications for the mechanism of HDL assembly.[Pubmed:27671775]
Biochim Biophys Acta. 2016 Dec;1861(12 Pt A):1968-1979.
ATP-binding cassette transporter A1 (ABCA1) mediates formation of disc-shaped high-density lipoprotein (HDL) from cell lipid and lipid-free apolipoprotein A-I (apo A-I). Discoidal HDL particles are heterogeneous in physicochemical characteristics for reasons that are understood incompletely. Discoidal lipoprotein particles similar in characteristics and heterogeneity to cell-formed discoidal HDL can be reconstituted from purified lipids and apo A-I by cell-free, physicochemical methods. The heterogeneity of reconstituted HDL (rHDL) is sensitive to the lipid composition of the starting lipid/apo A-I mixture. To determine whether the heterogeneity of cell-formed HDL is similarly sensitive to changes in cell lipids, we investigated four compounds that have well-established effects on cell lipid metabolism and ABCA1-mediated cell cholesterol efflux. 2-Bromopalmitate, D609, monensin and U18666A decreased formation of the larger-sized, but dramatically increased formation of the smaller-sized HDL. 2-Bromopalmitate did not appear to affect ABCA1 activity, subcellular localization or oligomerization, but induced dissolution of the cholesterol-phospholipid complexes in the plasma membrane. Arachidonic and linoleic acids shifted HDL formation to the smaller-sized species. Tangier disease mutations and inhibitors of ABCA1 activity wheat germ agglutinin and AG 490 reduced formation of both larger-sized and smaller-sized HDL. The effect of Probucol was similar to the effect of 2-bromopalmitate. Taking rHDL formation as a paradigm, we propose that ABCA1 mutations and activity inhibitors reduce the amount of cell lipid available for HDL formation, and the compounds in the 2-bromopalmitate group and the polyunsaturated fatty acids change cell lipid composition from one that favors formation of the larger-sized HDL particles to one that favors formation of the smaller-sized species.
Combination of cilostazol and probucol protected podocytes from lipopolysaccharide-induced injury by both anti-inflammatory and anti-oxidative mechanisms.[Pubmed:28005239]
J Nephrol. 2017 Aug;30(4):531-541.
Podocytes are essential for maintaining kidney glomerular functions. Injuries to podocyte are closely related to the pathological process of proteinuria. However, a treatment for podocyte injury has still not been established. Cilostazol (CSZ) and Probucol (PBC) have been shown to possess renoprotective effects. Therefore, we evaluated these drugs in a lipopolysaccharide (LPS)-induced podocyte injury model. 7-week-old female C57BL/6J mice were fed a normal diet or a diet containing 0.3% CSZ, 0.5% PBC, or both for 10 days. Then, mice were intraperitoneally injected with 13 mug g(-1) body weight LPS. Both CSZ and PBC decreased LPS-induced albuminuria and co-administration was found to be most effective. These treatments ameliorated the upregulation of monocyte chemoattractant protein 1. In cultured podocytes, CSZ suppressed LPS-induced activation of nuclear factor-kappa B (NF-kappaB) and phosphorylation of p44/42 mitogen-activated protein kinase (MAPK). PBC reduced LPS-induced activation of NF-kappaB and reactive oxygen species production. Furthermore, PBC decreased nicotinamide adenine dinucleotide phosphate (NADPH) oxidase4 expression. Our findings suggest that CSZ and PBC are able to inhibit podocyte-injury through different mechanisms, indicating that a combination of these two old drugs is a good treatment option to protect podocytes from injury.
Crystallization of probucol from solution and the glassy state.[Pubmed:27979761]
Int J Pharm. 2017 Jan 30;517(1-2):322-328.
Crystallization of Probucol (PBL) from both solution and glassy solid state was investigated. In the crystallization study from solution, six solvents and three methods, i.e., evaporation, addition of a poor solvent, and cooling on ice, were used to obtain various crystal forms. In addition to common two crystal forms (forms I and II), two further forms (forms III and cyclohexane-solvate) were found in this study, and their thermodynamic relationships were determined. Forms I and II are likely to be enantiotropically related with thermodynamic transition temperature below 5 degrees C. Isothermal crystallization studies revealed that PBL glass initially crystallized into form III between 25 and 50 degrees C, and then transformed to form I. The isothermal crystallization appears to be a powerful option to find uncommon crystal forms. The crystallization of PBL was identified to be pressure controlled, thus the physical stability of PBL glass is higher than that of typical compounds.
Probucol prevents atrial ion channel remodeling in an alloxan-induced diabetes rabbit model.[Pubmed:27863381]
Oncotarget. 2016 Dec 20;7(51):83850-83858.
Diabetes mellitus (DM) increases the risk of developing atrial fibrillation (AF), but the molecular mechanisms of diabetes-induced atrial remodeling processes have not been fully characterized. The aim of this study was to examine the mechanisms underlying atrial ion channel remodeling in alloxan-induced diabetes model in rabbits. A total of 40 Japanese rabbits were randomly assigned to a control group (C), alloxan-induced diabetic group (DM), Probucol-treated control group (Control-P), and Probucol-treated diabetic group (DM-P). Using whole-cell voltage-clamp techniques, ICa,L, INa and action potential durations (APDs) were measured in cardiomyocytes isolated from the left atria in the four groups, respectively. In the DM group, increased Ica,L and decreased INa currents were reflected in prolonged APD90 and APD50 values. These changes were reversed in the DM-P group. In conclusion, Probucol cured AF by alleviating the ion channel remodeling of atrial myocytes in the setting of diabetes and the promising therapeutic potential of anti-oxidative compounds in the treatment of AF warrants further study.
Probucol [4,4'-[(1-methylethylidene)bis(thio)]bis-[2,6-bis(1,1-dimethylethyl)phenol]] inhibits compensatory remodeling and promotes lumen loss associated with atherosclerosis in apolipoprotein E-deficient mice.[Pubmed:17293560]
J Pharmacol Exp Ther. 2007 May;321(2):477-84.
Probucol [4,4'-[(1-methylethylidene)bis(thio)]bis-[2,6-bis(1,1-dimethylethyl)phenol]] was withdrawn from the United States market because it failed to inhibit atherosclerosis in human femoral arteries, yet the drug was shown subsequently to inhibit atherosclerosis in human carotid arteries, and Probucol monosuccinate ester is presently being tested in a phase III clinical trial as an antiatherosclerotic compound based on its anti-inflammatory properties. Inflammatory macrophages are implicated in arterial remodeling associated with atherosclerosis, and Probucol inhibits experimental atherosclerosis in part by decreasing macrophages in lesions. However, the impact of Probucol on remodeling is unknown, although such knowledge could help explain why the drug's benefit on human atherosclerosis is controversial. We therefore examined the effect of Probucol on remodeling of the common carotid artery in apolipoprotein E-deficient mice. We observed that during de novo atherosclerosis, plaque growth was fully compensated by expansive remodeling, such that lumen area was unaffected. Early lesions were composed almost entirely of macrophages, and their contribution to lesion area progressively decreased thereafter. Probucol significantly decreased plaque area, expression of vascular cell adhesion molecule-1, and proliferation of intimal cells, resulting in delayed macrophage accumulation in the vessel. Probucol also decreased the production and activity of matrix metalloproteinases-2 and -9, independent of the plasmin protease system, and this was associated with an inhibition of expansive remodeling, resulting in lumen loss. These studies show that Probucol attenuates compensatory remodeling associated with de novo atherosclerosis, probably via its anti-inflammatory properties. Our findings suggest that lumen volume is not a suitable surrogate to assess the antiatherosclerotic activity of Probucol and related drugs.
Pretreatment with probucol attenuates cardiomyocyte apoptosis in a rabbit model of ischemia/reperfusion.[Pubmed:17101546]
Scand J Clin Lab Invest. 2006;66(7):549-58.
OBJECTIVE: Apoptosis plays an important role in ischemic reperfusion injury. Probucol is a hypolipidemic agent and has antioxidant activity, which may inhibit the oxidative modification of low-density lipoprotein cholesterol. Studies have demonstrated that Probucol improves left ventricular function, prevents left ventricular dilatation, and reduces cardiac fibrosis. However, the exact mechanism of Probucol on the cardioprotective effect is not known. The objective of the present study was to examine the effect of Probucol on ischemia/reperfusion-induced cardiomyocyte apoptosis. MATERIAL AND METHODS: Thirty male New Zealand White rabbits were randomly divided into sham, control, and treated groups, each group comprising 10 rabbits. Before establishment of the ischemia/reperfusion model, animals in the treated group were additionally fed daily with Probucol (1000 mg per day) for 4 weeks. In the sham group, the heart was exposed after the chest had been opened, but the coronary artery was not ligated. The animals were killed 150 min after the procedure. In the other two groups, the rabbits were subjected to 30-min of coronary occlusion followed by a 2-h reperfusion. A blood sample was drawn from the right atrium before the animal was killed. The apoptotic myocytes were detected by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling. Expression of caspase-3 and mitochondrial cytochrome c release was detected by immunohistochemical analysis and Western blot analysis. The level of serum superoxide dismutase (SOD) was tested using the xanthine oxidase method, and the content of serum malondialdehyde (MDA) was measured by colorimetry. RESULTS: As compared with the sham group, the control group had a significantly higher apoptotic index ((32.48 +/- 4.56) % versus (0.56 +/- 0.18) %, p < 0.01) and serum MDA concentration (2.70 +/- 0.64 versus 1.06 +/- 0.46 micromol/L, p < 0.01), and a significantly lower serum SOD level (144.27 +/- 21.69 versus 204.64 +/- 16.67 microU/L, p < 0.01). Probucol pretreatment apparently caused a decrease in the apoptotic index ((21.64 +/- 3.08) %, p < 0.01 versus the sham or control group) and serum MDA concentration (1.95 +/- 0.51 micromol/L, p < 0.01 versus the sham or control group), and increased the levels of serum SOD (162.61 +/- 16.13 microU/L, p < 0.01 versus the sham group; p < 0.05 versus the control group). The caspase-3 activation and mitochondrial cytochrome c release in the control group were also higher than those in the treated group (p < 0.01). CONCLUSIONS: The present study shows that Probucol attenuates ischemia/reperfusion-induced cardiomyocyte apoptosis. The protective effect of Probucol on the myocardium may be partly due to its antioxidant activity.
Ex vivo lipopolysaccharide-induced interleukin-1 secretion from murine peritoneal macrophages inhibited by probucol, a hypocholesterolemic agent with antioxidant properties.[Pubmed:2318380]
FASEB J. 1990 Apr 1;4(6):1645-53.
Probucol, 4,4'-(isopropylidenedithio)bis(2,6-di-tert-butyl-phenol), has been shown to inhibit atherogenesis in genetically hypercholesterolemic (Watanabe) rabbits. Since atherosclerotic lesions contain macrophages capable of screting interleukin 1 (IL 1) and other cytokines that could contribute to the pathogenesis of the disease, we have investigated whether Probucol affects IL 1 secretion. Resident peritoneal macrophages from mice dosed with Probucol secreted 40-80% less IL 1 than macrophages from control animals when stimulated in vitro with lipopolysaccharide (LPS). The inhibitory effect of Probucol was observed when IL 1 was assayed by the standard bioassay, the thymocyte proliferation assay, or a competitive IL 1 receptor binding assay. Probucol treatment had no effect on LPS-induced membrane IL 1 expression; secretion of tumor necrosis factor (TNF); Con A-induced splenic interleukin 2 (IL 2) and interleukin 3 (IL 3) release; and prostaglandin- or zymosan-induced secretion of prostacyclin, leukotriene C4, acid phosphatase, or superoxide anion. In contrast to the effect of oral administration, direct addition of Probucol to macrophage cultures did not inhibit IL 1 release. Probucol administration did, however, inhibit the fall in serum zinc level induced by intravenous injection of LPS in zymosan-primed mice but had no effect on the LPS-induced increase in serum triglyceride levels, which indirectly confirms that Probucol administration inhibits IL 1 but not TNF secretion. Paw granuloma induced in mice by heat-killed mycobacteria was inhibited by oral administration of Probucol, an effect that may be attributable to inhibition of IL 1 secretion. Probucol neither reduced zymosan-induced liver granulomata in mice nor inhibited adjuvant-induced arthritis in rats. We suggest that inhibition of IL 1 secretion from macrophages by Probucol contributes to its therapeutic effects in atherosclerosis and may also result in beneficial activity in some chronic inflammatory diseases.


