RiddellineCAS# 23246-96-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 23246-96-0 | SDF | Download SDF |
| PubChem ID | 5281744 | Appearance | White powder |
| Formula | C18H23NO6 | M.Wt | 349.38 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in chloroform | ||
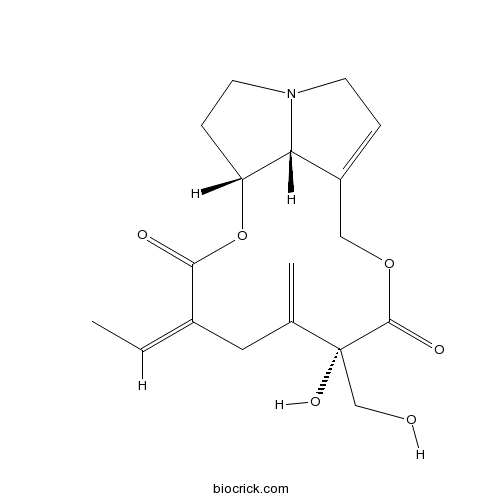
| SMILES | CC=C1CC(=C)C(C(=O)OCC2=CCN3C2C(CC3)OC1=O)(CO)O | ||
| Standard InChIKey | SVCNNZDUGWLODJ-RAYFHMIRSA-N | ||
| Standard InChI | InChI=1S/C18H23NO6/c1-3-12-8-11(2)18(23,10-20)17(22)24-9-13-4-6-19-7-5-14(15(13)19)25-16(12)21/h3-4,14-15,20,23H,2,5-10H2,1H3/b12-3-/t14-,15-,18-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Riddelline is a potent genotoxic agent in vitro and induces significant elevations in unscheduled DNA synthesis and S-phase synthesis in rat liver. |
| Targets | DNA/RNA Synthesis | VEGFR |

Riddelline Dilution Calculator

Riddelline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8622 mL | 14.3111 mL | 28.6221 mL | 57.2443 mL | 71.5553 mL |
| 5 mM | 0.5724 mL | 2.8622 mL | 5.7244 mL | 11.4489 mL | 14.3111 mL |
| 10 mM | 0.2862 mL | 1.4311 mL | 2.8622 mL | 5.7244 mL | 7.1555 mL |
| 50 mM | 0.0572 mL | 0.2862 mL | 0.5724 mL | 1.1449 mL | 1.4311 mL |
| 100 mM | 0.0286 mL | 0.1431 mL | 0.2862 mL | 0.5724 mL | 0.7156 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5,7-Diacetoxy-8-methoxyflavone
Catalog No.:BCN5083
CAS No.:23246-80-2
- Ritodrine HCl
Catalog No.:BCC4337
CAS No.:23239-51-2
- TR-14035
Catalog No.:BCC4266
CAS No.:232271-19-1
- 23-Hydroxymangiferonic acid
Catalog No.:BCN4668
CAS No.:232266-08-9
- Methoxydienone
Catalog No.:BCC9030
CAS No.:2322-77-2
- Doxorubicin
Catalog No.:BCC2082
CAS No.:23214-92-8
- Ifenprodil Tartrate
Catalog No.:BCC4589
CAS No.:23210-58-4
- Ifenprodil hemitartrate
Catalog No.:BCC6688
CAS No.:23210-56-2
- Matairesinoside
Catalog No.:BCN7583
CAS No.:23202-85-9
- (1R,2S)-2-Amino-1,2-diphenylethanol
Catalog No.:BCC8382
CAS No.:23190-16-1
- Columbianetin acetate
Catalog No.:BCN2652
CAS No.:23180-65-6
- Paeoniflorin
Catalog No.:BCN6301
CAS No.:23180-57-6
- Dimaprit dihydrochloride
Catalog No.:BCC6672
CAS No.:23256-33-9
- Guanabenz Acetate
Catalog No.:BCC4327
CAS No.:23256-50-0
- 4'-O-Methylvitexin
Catalog No.:BCN2642
CAS No.:2326-34-3
- Bay 36-7620
Catalog No.:BCC5915
CAS No.:232605-26-4
- VAL-083
Catalog No.:BCC2024
CAS No.:23261-20-3
- m-NH2-Tyr-OH.2HCl
Catalog No.:BCC3340
CAS No.:23279-22-3
- Probucol
Catalog No.:BCC4833
CAS No.:23288-49-5
- Delta 7-avenasterol
Catalog No.:BCN3212
CAS No.:23290-26-8
- Emodin-8-beta-D-glucoside
Catalog No.:BCN6329
CAS No.:23313-21-5
- Cephalexin monohydrate
Catalog No.:BCC4096
CAS No.:23325-78-2
- Nefopam HCl
Catalog No.:BCC4681
CAS No.:23327-57-3
- MK 0343
Catalog No.:BCC6170
CAS No.:233275-76-8
DNA cross-linking in mammalian cells by pyrrolizidine alkaloids: structure-activity relationships.[Pubmed:1949039]
Toxicol Appl Pharmacol. 1991 Oct;111(1):90-8.
Pyrrolizidine alkaloids (PAs) are common constituents of many species of flowering plants which possess carcinogenic as well as anticarcinogenic activity in vivo. Pyrrolizidine alkaloids are genotoxic in various short-term assays. The mechanisms by which these compounds exert these effects is still unclear. In this study, we characterized the ability of eight bifunctional PAs, with differing stereochemistry and functional groups, to cross-link cellular DNA in cultured bovine kidney epithelial cells. PAs representative of three major structural classes, the macrocycles (seneciphylline, Riddelline, retrorsine, senecionine, monocrotaline), the open diesters (heliosupine, latifoline), and pyrrolizidine base (retronecine) were cultured for 2 hr with cells and an external metabolizing system. Every PA induced DNA cross-links which consisted primarily of proteinase-sensitive cross-links (DPC), but also to a smaller extent, DNA interstrand cross-links (ISC). None of the PAs induced detectable amounts of DNA single-strand breaks. The PAs which produced DPC and/or ISC (ranked from most potent to least) were: seneciphylline (DPC greater than ISC); Riddelline (DPC greater than ISC); retrorsine (DPC greater than ISC); senecionine (DPC greater than ISC); heliosupine (DPC greater than ISC); monocrotaline (ISC = DPC); latifoline (DPC greater than ISC); and retronecine (ISC greater than DPC). Although the PAs induced DNA cross-linking to varying degrees, cell viabilities for all treatment groups were greater than 90% as determined by trypan blue dye exclusion. Since the cross-linking ability of these PAs paralleled their ability to inhibit colony formation, cross-link formation may be involved in the biological activity of these compounds. Two structural determinants of biological activity appear to be the presence of both a macrocyclic necic acid ester and an alpha,beta-unsaturated ester function since the cross-linking ability of seneciphylline, Riddelline, retrorsine, and senecionine far exceeded that of monocrotaline, heliosupine, latifoline, and retronecine. In addition, the stereochemical orientation of the ester linkage was found to have no effect on biological activity.
In vivo measurement of unscheduled DNA synthesis and S-phase synthesis as an indicator of hepatocarcinogenesis in rodents.[Pubmed:3507253]
Cell Biol Toxicol. 1987 Jun;3(2):165-73.
Measurement of chemically induced DNA repair as unscheduled DNA synthesis in rodent liver following in vivo treatment is a useful screen for potential hepatocarcinogens. In addition to measurement of unscheduled DNA synthesis, examination of S-phase synthesis provides an indicator of chemically induced cell proliferation in the liver, which may be a basis for hepatic tumor promotion. Several chemicals and classes of chemicals have been examined using these end points. The pyrrolizidine alkaloid Riddelline is a potent genotoxic agent in vitro, and in vivo studies confirm this response as Riddelline induces significant elevations in unscheduled DNA synthesis and S-phase synthesis in rat liver. Conversely, H.C. Blue dyes #1 and #2 are both potent genotoxic agents in vitro but fail to express this genotoxicity in vivo. H.C. Blue #1 induces significant increases in S-phase synthesis in B6C3F1 mouse liver, which correlates with the observed carcinogenicity of this compound. Halogenated hydrocarbons likewise fail to induce unscheduled DNA synthesis in vivo, but many of these compounds do increase hepatic cell proliferation in mice, which may be the principal mechanism of hepatocarcinogenesis in this species.
Analysis of vascular endothelial growth factor (VEGF) and a receptor subtype (KDR/flk-1) in the liver of rats exposed to riddelliine: a potential role in the development of hemangiosarcoma.[Pubmed:15384251]
Exp Toxicol Pathol. 2004 Jul;55(6):455-65.
Riddelliine alters hepatocellular and endothelial cell kinetics and function including stimulating an increase in hepatocytic vascular endothelial growth factor (VEGF) in the absence of increased serological levels of VEGF (NYSKA et al. 2002). The objective of this study was to further assess hepatic VEGF and KDR/flk-1 synthesis and expression by hepatic cells under riddelliine treatment conditions. Forty-two male F344/N rats were dosed by gavage with riddelliine (0, 1.0, and 2.5 mg/kg/day) for 6 weeks. Seven animals/group were sacrificed after 8 consecutive daily doses; remaining rats were terminated after 30 daily doses, excluding weekends. Hepatic tissues were evaluated by immunohistochemistry and in situ hybridization. The results showed that VEGF mRNA expression was observed in control and treated animals; however, qualitative differences were noted. Treated animals exhibited VEGF mRNA in clustered, focal hepatocytes and bile duct epithelium, whereas VEGF mRNA in hepatocytes from vehicle control rats was distributed evenly across all hepatocytes. Results evaluating the distribution of the VEGF cognate receptor, KDR/flk-1 showed that randomly distributed, rare sinusoidal endothelium, including those demonstrating karyomegaly and cytomegaly expressed KDR/flk-1. Phosphorylation of KDR/flk-1 at pTyr996 and pTyr1054/1059, but not pTyr951, was also detected, evidence that endothelial cell KDR/flk-1 was activated. These results suggest that both hepatocytes and endothelial cells are targets of riddelliine-induced injury. We speculate that damage to both populations of cells may lead to dysregulated VEGF synthesis by hepatocytes and activation of KDR/flk-1 by endothelium leading to the induction of sustained endothelial cell proliferation, culminating in the development of hepatic hemangiosarcoma.
Metabolic activation of the tumorigenic pyrrolizidine alkaloid, riddelliine, leading to DNA adduct formation in vivo.[Pubmed:11170513]
Chem Res Toxicol. 2001 Jan;14(1):101-9.
Riddelliine is a representative naturally occurring genotoxic pyrrolizidine alkaloid. We have studied the mechanism by which riddelliine induces hepatocellular tumors in vivo. Metabolism of riddelliine by liver microsomes of F344 female rats generated riddelliine N-oxide and dehydroretronecine (DHR) as major metabolites. Metabolism was enhanced when liver microsomes from phenobarbital-treated rats were used. Metabolism in the presence of calf thymus DNA resulted in eight DNA adducts that were identical to those obtained from the reaction of DHR with calf thymus DNA. Two of these adducts were identified as DHR-modified 7-deoxyguanosin-N(2)-yl epimers (DHR-3'-dGMP); the other six were DHR-derived DNA adducts, but their structures were not characterized. A similar DNA adduct profile was detected in the livers of female F344 rats fed riddelliine, and a dose-response relationship was obtained for the level of the total (eight) DHR-derived DNA adducts and the level of the DHR-3'-dGMP adducts. These results suggest that riddelliine induces liver tumors in rats through a genotoxic mechanism and the eight DHR-derived DNA adducts are likely to contribute to liver tumor development.


