GlycoborinineCAS# 233279-39-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 233279-39-5 | SDF | Download SDF |
| PubChem ID | 10446329 | Appearance | Powder |
| Formula | C18H17NO2 | M.Wt | 279.33 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
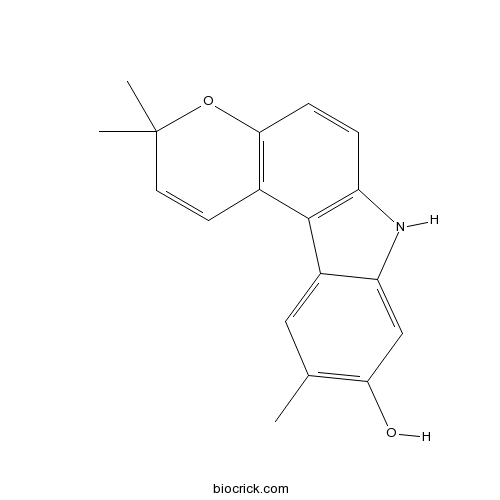
| Chemical Name | 3,3,10-trimethyl-7H-pyrano[3,2-g]carbazol-9-ol | ||
| SMILES | CC1=C(C=C2C(=C1)C3=C(N2)C=CC4=C3C=CC(O4)(C)C)O | ||
| Standard InChIKey | KGHHJTRYOSFBOZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H17NO2/c1-10-8-12-14(9-15(10)20)19-13-4-5-16-11(17(12)13)6-7-18(2,3)21-16/h4-9,19-20H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Glycoborinine is a potential molecule against cancer cells, it can induce HepG2 apoptosis through the mitochondrial-dependent pathway. 2. Glycoborinine has photo-activated antimicrobial activity, it shows moderate inhibition on Nde I, Xba I, Nco I and Bcl I. |
| Targets | ROS | Caspase | PARP |

Glycoborinine Dilution Calculator

Glycoborinine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.58 mL | 17.9 mL | 35.7999 mL | 71.5999 mL | 89.4999 mL |
| 5 mM | 0.716 mL | 3.58 mL | 7.16 mL | 14.32 mL | 17.9 mL |
| 10 mM | 0.358 mL | 1.79 mL | 3.58 mL | 7.16 mL | 8.95 mL |
| 50 mM | 0.0716 mL | 0.358 mL | 0.716 mL | 1.432 mL | 1.79 mL |
| 100 mM | 0.0358 mL | 0.179 mL | 0.358 mL | 0.716 mL | 0.895 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- MK 0343
Catalog No.:BCC6170
CAS No.:233275-76-8
- Nefopam HCl
Catalog No.:BCC4681
CAS No.:23327-57-3
- Cephalexin monohydrate
Catalog No.:BCC4096
CAS No.:23325-78-2
- Emodin-8-beta-D-glucoside
Catalog No.:BCN6329
CAS No.:23313-21-5
- Delta 7-avenasterol
Catalog No.:BCN3212
CAS No.:23290-26-8
- Probucol
Catalog No.:BCC4833
CAS No.:23288-49-5
- m-NH2-Tyr-OH.2HCl
Catalog No.:BCC3340
CAS No.:23279-22-3
- VAL-083
Catalog No.:BCC2024
CAS No.:23261-20-3
- Bay 36-7620
Catalog No.:BCC5915
CAS No.:232605-26-4
- 4'-O-Methylvitexin
Catalog No.:BCN2642
CAS No.:2326-34-3
- Guanabenz Acetate
Catalog No.:BCC4327
CAS No.:23256-50-0
- Dimaprit dihydrochloride
Catalog No.:BCC6672
CAS No.:23256-33-9
- L-Ser(Bzl)-ol
Catalog No.:BCC2579
CAS No.:23356-96-9
- Vinleurosine
Catalog No.:BCN2608
CAS No.:23360-92-1
- (1S,2R)-2-Amino-1,2-diphenylethanol
Catalog No.:BCC8385
CAS No.:23364-44-5
- Theviridoside
Catalog No.:BCN5084
CAS No.:23407-76-3
- Phalaenopsine T
Catalog No.:BCN2014
CAS No.:23412-97-7
- Phalaenopsine La
Catalog No.:BCN2015
CAS No.:23412-99-9
- 7-Isopentenyloxy-gamma-fagarine
Catalog No.:BCN5085
CAS No.:23417-92-7
- Tetrahydropiperin
Catalog No.:BCN6708
CAS No.:23434-88-0
- Swertianolin
Catalog No.:BCN2759
CAS No.:23445-00-3
- Irisolidone
Catalog No.:BCN8496
CAS No.:2345-17-7
- Physcion 1-glucoside
Catalog No.:BCN8170
CAS No.:23451-01-6
- Alternariol monomethyl ether
Catalog No.:BCN7384
CAS No.:23452-05-3
Photo-activated DNA binding and antimicrobial activities of alkaloids from Glycosmis pentaphylla.[Pubmed:23460971]
Yao Xue Xue Bao. 2012 Dec;47(12):1646-52.
In our screening for photosensitizers from natural resources, four alkaloids were isolated from Glycosmis pentaphylla by various chromatography techniques. Their structures were identified as Glycoborinine (1), glybomine B (2), carbalexin A (3) and N-p-coumaroyltyramine (4) by spectral analysis. Their photoactivated antimicrobial activities were evaluated by thin-layer chromatography (TLC) agar overlay assay against Staphylococcus aureus and Bacillus subtilis. It was found that compounds 1 and 4 showed photo-activated antimicrobial activities. Meantime, photo-activated DNA binding activities of these compounds were also assessed by using a specially prepared 1.8 kb DNA fragment and restriction enzymes. Under UVA irradiation, compound 1 showed moderate inhibition on Nde I, Xba I, Nco I and Bcl I which have either 5'-TpA or 5'-ApT and trace or no inhibition on other restriction enzymes. It showed a similar inhibition pattern with the reference 8-methoxypsoralen. However, compounds 2-4 showed no inhibition against any of the restriction enzymes.
Glycoborinine induces apoptosis through mitochondrial pathway in HepG2 cells.[Pubmed:24930917]
J Asian Nat Prod Res. 2014 Oct;16(10):991-9.
Glycoborinine (GB), a natural carbazole alkaloid isolated from Glycosmis pentaphylla, has been shown to be a potential molecule against cancer cells. In this study, the cell-signaling pathway of its anti-tumor activity was investigated. MTT assay result showed that GB inhibited HepG2 cell proliferation in a dose- and time-dependent manner and 50% inhibiting concentration (IC50) of GB-induced cell death was 39.7 muM for a period of 48 h. GB-induced HepG2 apoptosis was confirmed by Hochest 33258 staining and PI staining. The level of reactive oxygen species (ROS) was measured with H2DCF-DA staining and the change of mitochondrial membrane potential ( big up tri, openPsi(m)) was analyzed with tetrechloro-tetraethylbenzimidazolcarbocyanine iodide (JC-1) probe. Results showed that GB at 12.5, 25, and 50 muM promoted ROS production. GB induced HepG2 apoptosis through a mitochondrial apoptotic pathway, which was demonstrated by GB-induced increase in the ratio of Bax/Bcl-2, cytochrome C release, the ratio of cleaved caspase-3/procaspase-3, and the ratio of cleaved poly ADP-ribose polymerase (cleaved PARP)/poly ADP-ribose polymerase (PARP). To summarize, this study demonstrated that GB could induce HepG2 apoptosis through the mitochondrial-dependent pathway, which might provide a promising approach to cure liver cancer with GB.


