Alternariol monomethyl etherCAS# 23452-05-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 23452-05-3 | SDF | Download SDF |
| PubChem ID | 5360741 | Appearance | Powder |
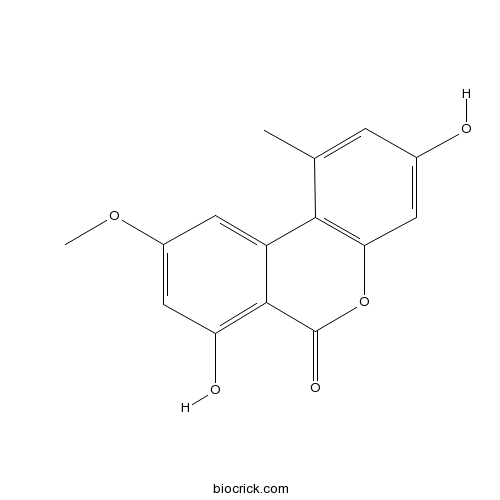
| Formula | C15H12O5 | M.Wt | 272.25 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3,7-dihydroxy-9-methoxy-1-methylbenzo[c]chromen-6-one | ||
| SMILES | CC1=CC(=CC2=C1C3=CC(=CC(=C3C(=O)O2)O)OC)O | ||
| Standard InChIKey | LCSDQFNUYFTXMT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H12O5/c1-7-3-8(16)4-12-13(7)10-5-9(19-2)6-11(17)14(10)15(18)20-12/h3-6,16-17H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Alternariol monomethyl ether,an Alternaria toxin, is a strong mutagen in Escherichia coli strain ND-160. 2. Alternariol monomethyl ether has genotoxic properties, it can cause oxidative DNA damage. 2. Alternariol monomethyl ether possesses cytotoxic and immunosuppressive properties. |
| Targets | Immunology & Inflammation related |

Alternariol monomethyl ether Dilution Calculator

Alternariol monomethyl ether Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6731 mL | 18.3655 mL | 36.7309 mL | 73.4619 mL | 91.8274 mL |
| 5 mM | 0.7346 mL | 3.6731 mL | 7.3462 mL | 14.6924 mL | 18.3655 mL |
| 10 mM | 0.3673 mL | 1.8365 mL | 3.6731 mL | 7.3462 mL | 9.1827 mL |
| 50 mM | 0.0735 mL | 0.3673 mL | 0.7346 mL | 1.4692 mL | 1.8365 mL |
| 100 mM | 0.0367 mL | 0.1837 mL | 0.3673 mL | 0.7346 mL | 0.9183 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Physcion 1-glucoside
Catalog No.:BCN8170
CAS No.:23451-01-6
- Irisolidone
Catalog No.:BCN8496
CAS No.:2345-17-7
- Swertianolin
Catalog No.:BCN2759
CAS No.:23445-00-3
- Tetrahydropiperin
Catalog No.:BCN6708
CAS No.:23434-88-0
- 7-Isopentenyloxy-gamma-fagarine
Catalog No.:BCN5085
CAS No.:23417-92-7
- Phalaenopsine La
Catalog No.:BCN2015
CAS No.:23412-99-9
- Phalaenopsine T
Catalog No.:BCN2014
CAS No.:23412-97-7
- Theviridoside
Catalog No.:BCN5084
CAS No.:23407-76-3
- (1S,2R)-2-Amino-1,2-diphenylethanol
Catalog No.:BCC8385
CAS No.:23364-44-5
- Vinleurosine
Catalog No.:BCN2608
CAS No.:23360-92-1
- L-Ser(Bzl)-ol
Catalog No.:BCC2579
CAS No.:23356-96-9
- Glycoborinine
Catalog No.:BCN7462
CAS No.:233279-39-5
- Trenbolone cyclohexylmethylcarbonate
Catalog No.:BCC9185
CAS No.:23454-33-3
- alpha-Spinasterone
Catalog No.:BCN5086
CAS No.:23455-44-9
- Decursinol
Catalog No.:BCN2638
CAS No.:23458-02-8
- trans-Khellactone
Catalog No.:BCN6920
CAS No.:23458-04-0
- 2-Palmitoylglycerol
Catalog No.:BCC7289
CAS No.:23470-00-0
- U 99194 maleate
Catalog No.:BCC7029
CAS No.:234757-41-6
- 2-Amino-5-mercapto-1,3,4-thiadiazole
Catalog No.:BCC8536
CAS No.:2349-67-9
- Hoechst 33258
Catalog No.:BCC1623
CAS No.:23491-45-4
- Hoechst 33342
Catalog No.:BCC1629
CAS No.:23491-52-3
- Hoechst 33258 analog 2
Catalog No.:BCC1625
CAS No.:23491-54-5
- Hoechst 33258 analog 5
Catalog No.:BCC1627
CAS No.:23491-55-6
- Peimine
Catalog No.:BCN1094
CAS No.:23496-41-5
The Alternaria alternata Mycotoxin Alternariol Suppresses Lipopolysaccharide-Induced Inflammation.[Pubmed:28726766]
Int J Mol Sci. 2017 Jul 20;18(7). pii: ijms18071577.
The Alternaria mycotoxins alternariol (AOH) and Alternariol monomethyl ether (AME) have been shown to possess genotoxic and cytotoxic properties. In this study, the ability of AOH and AME to modulate innate immunity in the human bronchial epithelial cell line (BEAS-2B) and mouse macrophage cell line (RAW264.7) were investigated. During these studies, it was discovered that AOH and to a lesser extent AME potently suppressed lipopolysaccharide (LPS)-induced innate immune responses in a dose-dependent manner. Treatment of BEAS-2B cells with AOH resulted in morphological changes including a detached pattern of growth as well as elongated arms. AOH/AME-related immune suppression and morphological changes were linked to the ability of these mycotoxins to cause cell cycle arrest at the G2/M phase. This model was also used to investigate the AOH/AME mechanism of immune suppression in relation to aryl hydrocarbon receptor (AhR). AhR was not found to be important for the immunosuppressive properties of AOH/AME, but appeared important for the low levels of cell death observed in BEAS-2B cells.
Minor contribution of alternariol, alternariol monomethyl ether and tenuazonic acid to the genotoxic properties of extracts from Alternaria alternata infested rice.[Pubmed:22906495]
Toxicol Lett. 2012 Oct 2;214(1):46-52.
Alternaria spp. are known to form a spectrum of secondary metabolites with alternariol (AOH), Alternariol monomethyl ether (AME), altenuene (ALT) and tenuazonic acid (TA) as the major mycotoxins with respect to quantity. In the present study we investigated the contribution of these compounds for the DNA damaging properties of complex extracts of Alternaria spp. infested rice. Five different Alternaria strains were cultured on rice and analyzed for their production of AOH, AME, ALT and TA. The extracts of two strains with distinctly different toxin profiles were selected for further toxicological analysis. An extract from A. alternata DSM 1102 infested rice, found to contain predominantly TA, exhibited substantial DNA strand breaking properties in cultured human colon carcinoma cells in the comet assay, whereas TA as a single compound did not affect DNA integrity up to 200muM. An extract of A. alternata DSM 12633 infested rice, containing in comparable proportions AOH, AME and TA, exceeded by far the DNA damaging properties of the single compounds. In contrast to AOH, AME and TA, both selected extracts induced an increase of DNA modifications sensitive to the bacterial repair enzyme formamidopyrimidine DNA glycosylase (FPG) in the comet assay, indicative for oxidative DNA damage. Toxicity-guided fractionation of the DSM 12633 extract confirmed that these effects were not caused by AOH, AME or TA. Taken together, the mycotoxins AOH, AME and TA, representing the major mycotoxins with respect to quantity in A. alternata infested food, play only a subordinate role for the genotoxic properties of complex extracts and appear not to be involved in the induction of FPG sensitive sites.


