Bay 65-1942 free baseIKKβ kinase inhibitor,ATP-competitive CAS# 600734-02-9 |
- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
- BMS-345541
Catalog No.:BCC1423
CAS No.:547757-23-3
- Bay 65-1942 HCl salt
Catalog No.:BCC1409
CAS No.:600734-06-3
- Bay 65-1942 R form
Catalog No.:BCC1410
CAS No.:758683-21-5
- Choline Fenofibrate
Catalog No.:BCC1478
CAS No.:856676-23-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 600734-02-9 | SDF | Download SDF |
| PubChem ID | 135454904 | Appearance | Powder |
| Formula | C22H25N3O4 | M.Wt | 395.45 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
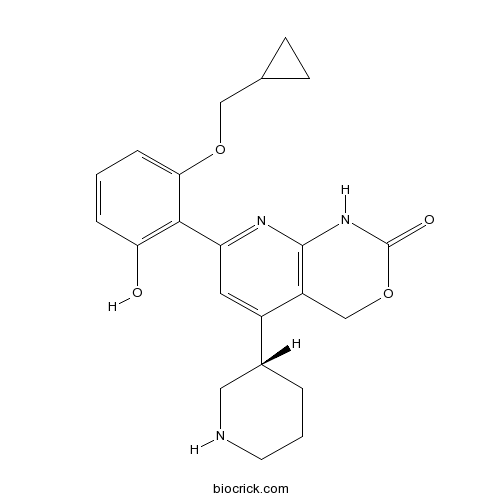
| Chemical Name | 7-[2-(cyclopropylmethoxy)-6-hydroxyphenyl]-5-[(3S)-piperidin-3-yl]-1,4-dihydropyrido[2,3-d][1,3]oxazin-2-one | ||
| SMILES | C1CC(CNC1)C2=CC(=NC3=C2COC(=O)N3)C4=C(C=CC=C4OCC5CC5)O | ||
| Standard InChIKey | IGJVFGZEWDGDOO-CQSZACIVSA-N | ||
| Standard InChI | InChI=1S/C22H25N3O4/c26-18-4-1-5-19(28-11-13-6-7-13)20(18)17-9-15(14-3-2-8-23-10-14)16-12-29-22(27)25-21(16)24-17/h1,4-5,9,13-14,23,26H,2-3,6-8,10-12H2,(H,24,25,27)/t14-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Bay 65-1942 free base Dilution Calculator

Bay 65-1942 free base Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5288 mL | 12.6438 mL | 25.2876 mL | 50.5753 mL | 63.2191 mL |
| 5 mM | 0.5058 mL | 2.5288 mL | 5.0575 mL | 10.1151 mL | 12.6438 mL |
| 10 mM | 0.2529 mL | 1.2644 mL | 2.5288 mL | 5.0575 mL | 6.3219 mL |
| 50 mM | 0.0506 mL | 0.2529 mL | 0.5058 mL | 1.0115 mL | 1.2644 mL |
| 100 mM | 0.0253 mL | 0.1264 mL | 0.2529 mL | 0.5058 mL | 0.6322 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Bay 65-1942 free base is a selective inhibitor of IKKβ with IC50 value of 10 μM and Ki value of 2 nM [1] [2].
IKKβ (inhibitor of nuclear factor kappa-B kinase subunit beta) is a subunit of IκB kinase which regulates the NF-κB activation and plays an important role in triggering immune responses [3].
Bay 65-1942 is a potent IKKβ inhibitor. When tested with MYL-R cells, administration of Bay 65-1942 reduced cell viability and induced cell apoptosis by decreasing IKKβ expression[1]. In human alveolar type II epithelium-like lung adenocarcinoma cell line A549, treatment of Bay 65-1942 inhibited IKKβ –dependent signal transduction and NF-κB activation by inhibiting IKKβ [2].
In acute ischemia-reperfusion C57BL/6 mice model, administration of Bay 65-1942 significantly reduced left ventricular infarct size and preserved cardiac function and finally prevented cardiac injury following IR by inhibiting IKKβ[4].
References:
[1.]Cooper, M.J., et al., Application of multiplexed kinase inhibitor beads to study kinome adaptations in drug-resistant leukemia. PLoS One, 2013. 8(6): p. e66755.
[2].Ziegelbauer, K., et al., A selective novel low-molecular-weight inhibitor of IkappaB kinase-beta (IKK-beta) prevents pulmonary inflammation and shows broad anti-inflammatory activity. Br J Pharmacol, 2005. 145(2): p. 178-92.
[3].Harken, A.H., The world of inhibitory kappaB. Am J Physiol Heart Circ Physiol, 2007. 293(5): p. H2624-5.
[4].Moss, N.C., et al., IKKbeta inhibition attenuates myocardial injury and dysfunction following acute ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol, 2007. 293(4): p. H2248-53.
- N-Demethylechitamine
Catalog No.:BCN4104
CAS No.:60048-88-6
- GL3
Catalog No.:BCN8180
CAS No.:60037-39-0
- Alizarin 2-methyl ether
Catalog No.:BCN3480
CAS No.:6003-11-8
- BOC-L-6-HYDROXYNORLEUCINE
Catalog No.:BCN2360
CAS No.:77611-37-1
- Chlormethiazole hydrochloride
Catalog No.:BCC6830
CAS No.:6001-74-7
- Glabrene
Catalog No.:BCN6692
CAS No.:60008-03-9
- Oblongine
Catalog No.:BCN4103
CAS No.:60008-01-7
- 11Beta-hydroxyprogesterone
Catalog No.:BCN2211
CAS No.:600-57-7
- N-Me-DL-Ala-OH.HCl
Catalog No.:BCC2618
CAS No.:600-21-5
- Adenosine cyclophosphate
Catalog No.:BCN2190
CAS No.:60-92-4
- Phloretin
Catalog No.:BCN4128
CAS No.:60-82-2
- Phlorizin
Catalog No.:BCN4126
CAS No.:60-81-1
- Bay 65-1942 HCl salt
Catalog No.:BCC1409
CAS No.:600734-06-3
- Secologanic acid
Catalog No.:BCN8214
CAS No.:60077-46-5
- Vogeloside
Catalog No.:BCN6737
CAS No.:60077-47-6
- (1)Benzopyrano(3,4-b)furo(2,3-h)(1)benzopyran-6(12H)-one, 1,2,-dihydro-5-hydroxy-8,9-dimethoxy-2-(1-methylethenyl)-, (R)-
Catalog No.:BCN8538
CAS No.:60077-62-5
- Confluentic acid
Catalog No.:BCN4105
CAS No.:6009-12-7
- Sodium taurochenodeoxycholate
Catalog No.:BCN8152
CAS No.:6009-98-9
- Beta-D-glucopyranosiduronic acid
Catalog No.:BCN3251
CAS No.:60092-34-4
- Thamnosmonin
Catalog No.:BCN6931
CAS No.:60094-90-8
- Isosativan
Catalog No.:BCN4106
CAS No.:60102-29-6
- Petasitenine
Catalog No.:BCN2113
CAS No.:60102-37-6
- Baimantuoluoside C
Catalog No.:BCN8009
CAS No.:60124-17-6
- 3-(2-Hydroxyphenyl)-2-propenal
Catalog No.:BCN4107
CAS No.:60125-23-7
Marked differences in base selectivity between DNA and the free nucleotides upon adduct formation from Bay- and Fjord-region diol epoxides.[Pubmed:10995261]
Chem Res Toxicol. 2000 Sep;13(9):883-90.
Distributions of adducts formed from each of the four optically active isomers of 3,4-dihydroxy-1,2-epoxy-1,2,3, 4-tetrahydrobenzo[c]phenanthrene and of 7,8-dihydroxy-9,10-epoxy-7,8, 9,10- tetrahydrobenzo[a]pyrene (BcPh and BaP diol epoxides) on reaction with an equimolar mixture of deoxyadenosine and deoxyguanosine 5'-monophosphates were compared with the known adduct distributions from these diol epoxides (DEs) upon reaction with calf thymus DNA in vitro. In the presence of an equimolar (100 mM total) mixture of dAMP and dGMP, the efficiency of formation of all types of adducts relative to tetraols is comparable for both the BaP ( approximately 40-60%) and BcPh ( approximately 30-40%) diol epoxides. This is in contrast to the partitioning between tetraols and adducts observed with DNA, where the BcPh DEs form adducts much more efficiently than the BaP DEs. Preference for trans versus cis ring opening by the exocyclic amino groups of the free nucleotides in the dAMP/dGMP mixture is greater for the DE diastereomer in which the benzylic hydroxyl group and the epoxide oxygen are trans (DE-2). This is qualitatively similar to the preferences for trans versus cis adduct formation on reaction of these isomers with DNA, as well as trans versus cis tetraol formation on their acid hydrolysis. For the BcPh DE isomers, competitive reaction between dGMP and dAMP gives 40-62% of the total exocyclic amino group adducts as dA adducts. A similar distribution of dG versus dA adducts had previously been observed on reaction of the BcPh DEs with DNA, except in the case of (+)-3(R),4(S)-dihydroxy-1(R),2(S)-epoxy-1,2,3, 4-tetrahydrobenzo[c]phenanthrene, which gives approximately 85% dA adducts on reaction with DNA. With the BaP DEs, 60-77% of the exocyclic amino group adducts formed upon competitive reaction with the free nucleotides are derived from dGMP. The observed dG selectivity of these BaP DEs is much smaller with the nucleotide mixture than it is with DNA, leading to the conclusion that DNA structure has a much larger modifying effect on the base selectivity of the BaP relative to the BcPh DEs.


