Secologanic acidCAS# 60077-46-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 60077-46-5 | SDF | Download SDF |
| PubChem ID | 71607801 | Appearance | Powder |
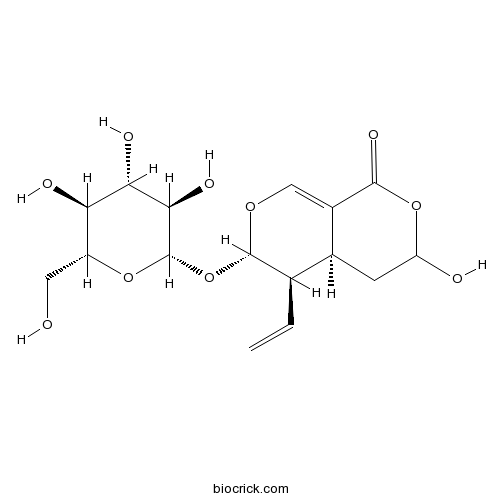
| Formula | C16H22O10 | M.Wt | 374.3 |
| Type of Compound | Iridoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (4aS,5R,6S)-5-ethenyl-3-hydroxy-6-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4,4a,5,6-tetrahydro-3H-pyrano[3,4-c]pyran-1-one | ||
| SMILES | C=CC1C2CC(OC(=O)C2=COC1OC3C(C(C(C(O3)CO)O)O)O)O | ||
| Standard InChIKey | LCTYGGXMQYUYHL-UEMOXOMHSA-N | ||
| Standard InChI | InChI=1S/C16H22O10/c1-2-6-7-3-10(18)25-14(22)8(7)5-23-15(6)26-16-13(21)12(20)11(19)9(4-17)24-16/h2,5-7,9-13,15-21H,1,3-4H2/t6-,7+,9-,10?,11-,12+,13-,15+,16+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Secologanic acid shows inhibition of nitric oxide production in lipopolysaccharide-activated macrophages. 2. Secologanic acid is a plant growth inhibitor. |
| Targets | NO |

Secologanic acid Dilution Calculator

Secologanic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6717 mL | 13.3583 mL | 26.7165 mL | 53.4331 mL | 66.7913 mL |
| 5 mM | 0.5343 mL | 2.6717 mL | 5.3433 mL | 10.6866 mL | 13.3583 mL |
| 10 mM | 0.2672 mL | 1.3358 mL | 2.6717 mL | 5.3433 mL | 6.6791 mL |
| 50 mM | 0.0534 mL | 0.2672 mL | 0.5343 mL | 1.0687 mL | 1.3358 mL |
| 100 mM | 0.0267 mL | 0.1336 mL | 0.2672 mL | 0.5343 mL | 0.6679 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Bay 65-1942 HCl salt
Catalog No.:BCC1409
CAS No.:600734-06-3
- Bay 65-1942 free base
Catalog No.:BCC1408
CAS No.:600734-02-9
- N-Demethylechitamine
Catalog No.:BCN4104
CAS No.:60048-88-6
- GL3
Catalog No.:BCN8180
CAS No.:60037-39-0
- Alizarin 2-methyl ether
Catalog No.:BCN3480
CAS No.:6003-11-8
- BOC-L-6-HYDROXYNORLEUCINE
Catalog No.:BCN2360
CAS No.:77611-37-1
- Chlormethiazole hydrochloride
Catalog No.:BCC6830
CAS No.:6001-74-7
- Glabrene
Catalog No.:BCN6692
CAS No.:60008-03-9
- Oblongine
Catalog No.:BCN4103
CAS No.:60008-01-7
- 11Beta-hydroxyprogesterone
Catalog No.:BCN2211
CAS No.:600-57-7
- N-Me-DL-Ala-OH.HCl
Catalog No.:BCC2618
CAS No.:600-21-5
- Adenosine cyclophosphate
Catalog No.:BCN2190
CAS No.:60-92-4
- Vogeloside
Catalog No.:BCN6737
CAS No.:60077-47-6
- (1)Benzopyrano(3,4-b)furo(2,3-h)(1)benzopyran-6(12H)-one, 1,2,-dihydro-5-hydroxy-8,9-dimethoxy-2-(1-methylethenyl)-, (R)-
Catalog No.:BCN8538
CAS No.:60077-62-5
- Confluentic acid
Catalog No.:BCN4105
CAS No.:6009-12-7
- Sodium taurochenodeoxycholate
Catalog No.:BCN8152
CAS No.:6009-98-9
- Beta-D-glucopyranosiduronic acid
Catalog No.:BCN3251
CAS No.:60092-34-4
- Thamnosmonin
Catalog No.:BCN6931
CAS No.:60094-90-8
- Isosativan
Catalog No.:BCN4106
CAS No.:60102-29-6
- Petasitenine
Catalog No.:BCN2113
CAS No.:60102-37-6
- Baimantuoluoside C
Catalog No.:BCN8009
CAS No.:60124-17-6
- 3-(2-Hydroxyphenyl)-2-propenal
Catalog No.:BCN4107
CAS No.:60125-23-7
- Paniculoside I
Catalog No.:BCN4108
CAS No.:60129-63-7
- Paniculoside II
Catalog No.:BCN4109
CAS No.:60129-64-8
Influence of sulfur fumigation on the chemical constituents and antioxidant activity of buds of Lonicera japonica.[Pubmed:25342552]
Molecules. 2014 Oct 15;19(10):16640-55.
Lonicera japonica flos is widely used as a pharmaceutical resource and a commonly-employed ingredient in healthy food, soft beverages and cosmetics in China. Sometimes, sulfur fumigation is used during post-harvest handling. In this study, a comprehensive comparison of the chemical profile between sun-dried and sulfur-fumigated samples was conducted by HPLC fingerprints and simultaneous quantification of nine constituents, including Secologanic acid, along with another eight usually-analyzed markers. Secologanic acid was destroyed, and its sulfonates were generated, whereas caffeoylquinic acids were protected from being oxidized. The residual sulfur dioxide in sulfur-fumigated samples was significantly higher than that in sun-dried samples, which might increase the potential incidence of toxicity to humans. Meanwhile, compared with sun-dried samples, sulfur-fumigated samples have significantly stronger antioxidant activity, which could be attributed to the joint effect of protected phenolic acids and flavonoids, as well as newly-generated iridoid sulfonates.
Allelochemicals of the tropical weed Sphenoclea zeylanica.[Pubmed:11065289]
Phytochemistry. 2000 Sep;55(2):131-40.
Nine plant growth inhibitors were isolated from the tropical weed Sphenoclea zeylanica, which shows allelopathic properties. Those compounds hitherto not reported from any plant source were the isomers of cyclic thiosulfinate, (1S,3R,4R)-(+)- and (1R,3R,4R)-(+)-4-hydroxy-3-hydroxymethyl-1,2-dithiolane-1-oxides, and (2R,3R,4R)-(-)- and (2S,3R,4R)-(+)-4-hydroxy-3-hydroxymethyl-1,2-dithiolane-2-oxides. These were named zeylanoxide A, epi-zeylanoxide A, zeylanoxide B and epi-zeylanoxide B, respectively. The absolute configurations at C-3 and C-4 were elucidated by chemical synthesis of both enantiomers from L- and D-glucose. Two of the inhibitors were Secologanic acid and secologanoside. and three other inhibitors were by known secoiridoid glucosides formed as artifacts during extraction with methanol. The cyclic thiosulfinates and secoiridoid glucosides completely inhibit the root growth of rice seedlings at 3.0 mM. While the specific activity of the inhibitors was not high, since they accumulated to circa 0.61% S. zelanica by dry weight, this suggests that the inhibitors are nervertheless potent allelochemicals in this weed.
Modified secoiridoid from Acicarpha tribuloides and inhibition of nitric oxide production in LPS-activated macrophages.[Pubmed:16808936]
Phytochemistry. 2006 Jul;67(14):1534-8.
Bioassay-guided fractionation of Acicarpha tribuloides Juss. resulted in the isolation of an uncommon non-glycosylated secoiridoid, tribulolide (1), two known secoiridoid glycosides named Secologanic acid (2) and vogeloside (3) as well as two natural chromones, 6,7-dimethoxychromone (4) and 7-hydroxy-6-methoxy-chromone (5). Compounds 1-3 showed inhibition of nitric oxide production in lipopolysaccharide-activated macrophages; their activity is comparable to that of aminoguanidine, a classic inhibitor.


