Paniculoside ICAS# 60129-63-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 60129-63-7 | SDF | Download SDF |
| PubChem ID | 14396747 | Appearance | Powder |
| Formula | C26H40O8 | M.Wt | 480.6 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
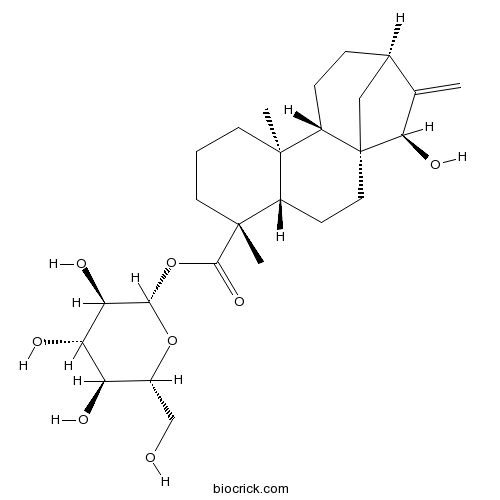
| SMILES | CC12CCCC(C1CCC34C2CCC(C3)C(=C)C4O)(C)C(=O)OC5C(C(C(C(O5)CO)O)O)O | ||
| Standard InChIKey | RSQGPCRWQCUQBR-HLTNFVLASA-N | ||
| Standard InChI | InChI=1S/C26H40O8/c1-13-14-5-6-17-24(2)8-4-9-25(3,16(24)7-10-26(17,11-14)21(13)31)23(32)34-22-20(30)19(29)18(28)15(12-27)33-22/h14-22,27-31H,1,4-12H2,2-3H3/t14-,15-,16+,17+,18-,19+,20-,21-,22+,24-,25-,26-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Paniculoside I is a natural product from Stevia rebaudiana. |
| Structure Identification | Chemical & Pharmaceutical Bulletin , 2008 , 25 (11) :2895-2899.Application of ^<13>C Nuclear Magnetic Resonance Spectroscopy to Chemistry of Glycosides : Structures of Paniculosides-I, -II, -III, -IV, and -V, Diterpene Glucosides of Stevia paniculata LAG.[Reference: WebLink]From the aerial part of Stevia paniculata (Compositae), five new diterpene glucosides, named Paniculoside I, Paniculoside II, Paniculoside III, Paniculoside IV, and Paniculoside V(5-9) were isolated.
|

Paniculoside I Dilution Calculator

Paniculoside I Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0807 mL | 10.4037 mL | 20.8073 mL | 41.6146 mL | 52.0183 mL |
| 5 mM | 0.4161 mL | 2.0807 mL | 4.1615 mL | 8.3229 mL | 10.4037 mL |
| 10 mM | 0.2081 mL | 1.0404 mL | 2.0807 mL | 4.1615 mL | 5.2018 mL |
| 50 mM | 0.0416 mL | 0.2081 mL | 0.4161 mL | 0.8323 mL | 1.0404 mL |
| 100 mM | 0.0208 mL | 0.104 mL | 0.2081 mL | 0.4161 mL | 0.5202 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3-(2-Hydroxyphenyl)-2-propenal
Catalog No.:BCN4107
CAS No.:60125-23-7
- Baimantuoluoside C
Catalog No.:BCN8009
CAS No.:60124-17-6
- Petasitenine
Catalog No.:BCN2113
CAS No.:60102-37-6
- Isosativan
Catalog No.:BCN4106
CAS No.:60102-29-6
- Thamnosmonin
Catalog No.:BCN6931
CAS No.:60094-90-8
- Beta-D-glucopyranosiduronic acid
Catalog No.:BCN3251
CAS No.:60092-34-4
- Sodium taurochenodeoxycholate
Catalog No.:BCN8152
CAS No.:6009-98-9
- Confluentic acid
Catalog No.:BCN4105
CAS No.:6009-12-7
- (1)Benzopyrano(3,4-b)furo(2,3-h)(1)benzopyran-6(12H)-one, 1,2,-dihydro-5-hydroxy-8,9-dimethoxy-2-(1-methylethenyl)-, (R)-
Catalog No.:BCN8538
CAS No.:60077-62-5
- Vogeloside
Catalog No.:BCN6737
CAS No.:60077-47-6
- Secologanic acid
Catalog No.:BCN8214
CAS No.:60077-46-5
- Bay 65-1942 HCl salt
Catalog No.:BCC1409
CAS No.:600734-06-3
- Paniculoside II
Catalog No.:BCN4109
CAS No.:60129-64-8
- Paniculoside III
Catalog No.:BCN7503
CAS No.:60129-65-9
- Pterodondiol
Catalog No.:BCN4110
CAS No.:60132-35-6
- Ercalcitriol
Catalog No.:BCC1556
CAS No.:60133-18-8
- Cucurbitacin S
Catalog No.:BCN2676
CAS No.:60137-06-6
- Coulteropine
Catalog No.:BCN7420
CAS No.:6014-62-6
- Cephaelin dihydrobromide
Catalog No.:BCC8142
CAS No.:6014-81-9
- Gabapentin HCl
Catalog No.:BCC4502
CAS No.:60142-95-2
- Gabapentin
Catalog No.:BCC3783
CAS No.:60142-96-3
- 11,12-Dihydro-7-hydroxyhedychenone
Catalog No.:BCN7382
CAS No.:60149-07-7
- TWS119
Catalog No.:BCC4512
CAS No.:601514-19-6
- Sodium 4-Aminosalicylate
Catalog No.:BCC4609
CAS No.:6018-19-5
Phytochemical, antimicrobial and antiquorum-sensing studies of pulicaria undulata L.: a revision on the structure of 1beta,2alpha,3beta,19alpha,23-pentahydroxy-urs-12-en-28-oic acid.[Pubmed:30422011]
Nat Prod Res. 2018 Nov 13:1-6.
Phytochemical study of the aerial part of Pulicaria undulata L. led to the isolation of nine compounds. The structure of 1beta,2alpha,3beta,19alpha,23-pentahydroxy-urs-12-en-28-oic acid (4) was revised and confirmation of the stereochemical configuration of the hydroxyl groups was established using NOESY and selective decoupling experiments. The other compounds were identified as 1,2-dehydro-1,10alpha-dihydropseudoivalin (1), axillarin (2), grandifloric acid-15-beta-glucoside (3), myrianthic acid (5), caffeic acid (6), quercetin (7), Paniculoside IV (8) and caffeic anhydride (9). The structures were characterized by 1 D, 2 D NMR spectroscopy and confirmed with HRMS. Antimicrobial and antiquorum-sensing activities of the different extracts and isolated compounds of the plant were investigated. Generally, the phenolic rather than the terpenoidal compounds exhibited remarkable antimicrobial and antiquorum-sensing activity. [Formula: see text].
New ent-kauranes from the fruits of Annona glabra and their inhibitory nitric oxide production in LPS-stimulated RAW264.7 macrophages.[Pubmed:25499882]
Bioorg Med Chem Lett. 2015 Jan 15;25(2):254-8.
Three new ent-kaurane diterpenoids, 7beta,16alpha,17-trihydroxy-ent-kauran-19-oic acid (1), 7beta,17-dihydroxy-16alpha-ent-kauran-19-oic acid 19-O-beta-d-glucopyranoside ester (2), 7beta,17-dihydroxy-ent-kaur-15-en-19-oic acid 19-O-beta-d-glucopyranoside ester (3) along with five known compounds, Paniculoside IV (4), 16alpha,17-dihydroxy-ent-kaurane (5), 16beta,17-dihydroxy-ent-kaurane (6), 16beta,17-dihydroxy-ent-kauran-19-al (7), and 16beta,17-dihydroxy-ent-kauran-19-oic acid (8) were isolated from the fruits of Annona glabra. Their chemical structures were elucidated by physical and chemical methods. All compounds were evaluated for inhibitory activity against nitric oxide (NO) production in LPS-stimulated RAW 264.7 macrophages. As the results, compound 3 showed potent inhibitory LPS-stimulated NO production in RAW 264.7 macrophages with the IC50 value of 0.01+/-0.01muM; compounds 1 and 7 showed significant inhibitory NO production with the IC50 values of 0.39+/-0.12muM and 0.32+/-0.04muM, respectively.
Helikauranoside a, a new bioactive diterpene.[Pubmed:18071822]
J Chem Ecol. 2008 Jan;34(1):65-9.
A new ent-kaurane glucoside, named helikauranoside A (4), was isolated from the aerial parts of Helianthus annuus L. together with three known ent-kaurane-type diterpenoids: (-)-kaur-16-en-19-oic acid (1), grandifloric acid (2), and Paniculoside IV (3). The structure of 4 was determined by using a combination of 1D (1H-NMR and 13C-NMR) and 2D (COSY, HSQC, and HMBC) NMR techniques. Bioactivity spectra of isolated compounds were tested by using the etiolated wheat coleoptile bioassay in aqueous solutions at concentrations ranging from 10(-3) to 10(-6)M. Helikauranoside A (4) was the most active (-84%, 10(-3)M; -56%, 10(-4)M). These results suggest that this new compound may be involved in defense mechanisms of H. annuus.
A new ent-kaurane diterpenoid glycoside from the leaves of Cussonia bojeri, a Malagasy endemic plant.[Pubmed:12192150]
Chem Pharm Bull (Tokyo). 2002 Aug;50(8):1122-3.
A new ent-kaurane diterpene glycoside, beta-D-glucopyranosyl 17-hydroxy-ent-kauran-19-oate-16-O-beta-D-glucopyranoside (4) was isolated from the dried leaves of Cussonia bojeri SEEM., together with four known compounds identified as 16beta,17-dihydroxy-kauran-19-oic acid (1), beta-D-glucopyranosyl 16beta,17-dihydroxy-(-)-kauran-19-oate (2), Paniculoside IV (3), and rutin (5). The structure of 4 was deduced on the basis of chemical and spectroscopic evidence.
Ent-kaurane diterpenoid glycosides from the leaves of Cussonia racemosa, a Malagasy endemic plant.[Pubmed:11848221]
Chem Pharm Bull (Tokyo). 2002 Feb;50(2):268-71.
Six new ent-kaurane diterpenoid glycosides, cussoracosides A (3), B (4), C (5), D (6), E (7), and F (8) were isolated from the dried leaves of Cussonia racemosa, along with two known compounds identified as beta-D-glucopyranosyl ent-16beta,17-dihydroxykauran-19-oate (1) and Paniculoside IV (2). The structures of these new compounds were deduced on the basis of chemical and spectroscopic evidence.


