PterodondiolCAS# 60132-35-6 |
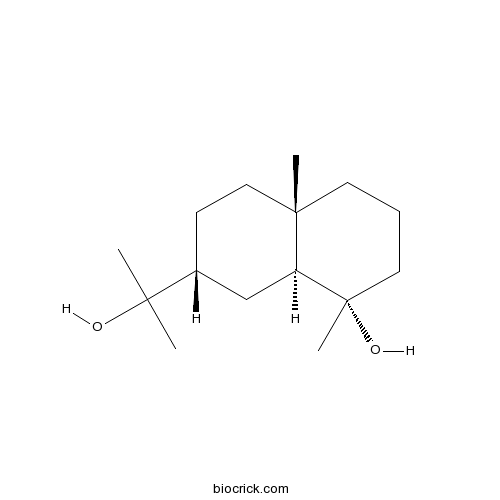
- Cryptomeridiol
Catalog No.:BCN5516
CAS No.:4666-84-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 60132-35-6 | SDF | Download SDF |
| PubChem ID | 10879263 | Appearance | Cryst. |
| Formula | C15H28O2 | M.Wt | 240.4 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1S,4aS,7R,8aS)-7-(2-hydroxypropan-2-yl)-1,4a-dimethyl-2,3,4,5,6,7,8,8a-octahydronaphthalen-1-ol | ||
| SMILES | CC12CCCC(C1CC(CC2)C(C)(C)O)(C)O | ||
| Standard InChIKey | LKKDASYGWYYFIK-DHMWGJHJSA-N | ||
| Standard InChI | InChI=1S/C15H28O2/c1-13(2,16)11-6-9-14(3)7-5-8-15(4,17)12(14)10-11/h11-12,16-17H,5-10H2,1-4H3/t11-,12+,14+,15+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Pterodondiol has moderate activity against bacteria including Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus subtilis, Mycobacteium phlei and Bacillus circulans. |
| Targets | Antifection |

Pterodondiol Dilution Calculator

Pterodondiol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1597 mL | 20.7987 mL | 41.5973 mL | 83.1947 mL | 103.9933 mL |
| 5 mM | 0.8319 mL | 4.1597 mL | 8.3195 mL | 16.6389 mL | 20.7987 mL |
| 10 mM | 0.416 mL | 2.0799 mL | 4.1597 mL | 8.3195 mL | 10.3993 mL |
| 50 mM | 0.0832 mL | 0.416 mL | 0.8319 mL | 1.6639 mL | 2.0799 mL |
| 100 mM | 0.0416 mL | 0.208 mL | 0.416 mL | 0.8319 mL | 1.0399 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Paniculoside III
Catalog No.:BCN7503
CAS No.:60129-65-9
- Paniculoside II
Catalog No.:BCN4109
CAS No.:60129-64-8
- Paniculoside I
Catalog No.:BCN4108
CAS No.:60129-63-7
- 3-(2-Hydroxyphenyl)-2-propenal
Catalog No.:BCN4107
CAS No.:60125-23-7
- Baimantuoluoside C
Catalog No.:BCN8009
CAS No.:60124-17-6
- Petasitenine
Catalog No.:BCN2113
CAS No.:60102-37-6
- Isosativan
Catalog No.:BCN4106
CAS No.:60102-29-6
- Thamnosmonin
Catalog No.:BCN6931
CAS No.:60094-90-8
- Beta-D-glucopyranosiduronic acid
Catalog No.:BCN3251
CAS No.:60092-34-4
- Sodium taurochenodeoxycholate
Catalog No.:BCN8152
CAS No.:6009-98-9
- Confluentic acid
Catalog No.:BCN4105
CAS No.:6009-12-7
- (1)Benzopyrano(3,4-b)furo(2,3-h)(1)benzopyran-6(12H)-one, 1,2,-dihydro-5-hydroxy-8,9-dimethoxy-2-(1-methylethenyl)-, (R)-
Catalog No.:BCN8538
CAS No.:60077-62-5
- Ercalcitriol
Catalog No.:BCC1556
CAS No.:60133-18-8
- Cucurbitacin S
Catalog No.:BCN2676
CAS No.:60137-06-6
- Coulteropine
Catalog No.:BCN7420
CAS No.:6014-62-6
- Cephaelin dihydrobromide
Catalog No.:BCC8142
CAS No.:6014-81-9
- Gabapentin HCl
Catalog No.:BCC4502
CAS No.:60142-95-2
- Gabapentin
Catalog No.:BCC3783
CAS No.:60142-96-3
- 11,12-Dihydro-7-hydroxyhedychenone
Catalog No.:BCN7382
CAS No.:60149-07-7
- TWS119
Catalog No.:BCC4512
CAS No.:601514-19-6
- Sodium 4-Aminosalicylate
Catalog No.:BCC4609
CAS No.:6018-19-5
- Arecaidine hydrochloride
Catalog No.:BCN8530
CAS No.:6018-28-6
- Corypalmine
Catalog No.:BCN4111
CAS No.:6018-40-2
- Methyl 15-hydroxy-7-oxodehydroabietate
Catalog No.:BCN7674
CAS No.:60188-95-6
Cytotoxic and antioxidant compounds from the stem bark of Goniothalamus tapisoides Mat Salleh.[Pubmed:23344192]
Molecules. 2012 Dec 21;18(1):128-39.
Eleven compounds:goniomicin A (1), goniomicin B (2), goniomicin C (3), goniomicin D (4), tapisoidin (5), goniothalamin (6), 9-deoxygoniopypyrone (7), Pterodondiol (8), liriodenine (9), benzamide (10) and cinnamic acid (11), were isolated from the stem bark of Goniothalamus tapisoides. All compounds were identified by spectroscopic analysis and, for known compounds, by comparison with published data. Goniothalamin (6) exhibited mild cytotoxic activity towards a colon cancer cell line (HT-29), with an IC(50)value of 64.17 +/- 5.60 microM. Goniomicin B (2) give the highest antioxidant activity in the DPPH assay among all compounds tested, with an IC(50) of 0.207 microM.
Terpenoids and flavonoids from Laggera pterodonta.[Pubmed:17703774]
Yao Xue Xue Bao. 2007 May;42(5):511-5.
To study the chemical constituents of aerial parts of Laggera pterodonta (DC.) Benth., the air-dried aerial parts of this plant were powered and extracted with boiling water and purified by silica gel column chromatography and recrystallized. Eleven compounds were obtained from L. pterodonta. They were identified as to be 6-O-beta-D-glucopyranosyl-carvotanacetone (1), pterodontic acid (2), 1beta-hydroxy pterondontic acid (3), pterodontoside A (4), Pterodondiol (5), pterodontriol B (6), 5-hydroxy-3,4', 6,7-tetramethoxyflavone (7), artemitin (8), chrysosplenetin B (9), quercetin (10) and beta-sitosterol (11). Compound 1 is a new monoterpene glucoside. Compounds 10 and 11 were isolated from this plant for the first time. Compounds 2 and 5 showed moderate activity against bacteria including Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus subtilis, Mycobacteium phlei and Bacillus circulans by paper disc diffusion method, while they both displayed no activity against Escherichia coli.


