H-Tyr(tBu)-OHCAS# 18822-59-8 |
- H-D-Tyr(tBu)-OH
Catalog No.:BCC3137
CAS No.:186698-58-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 18822-59-8 | SDF | Download SDF |
| PubChem ID | 7408092 | Appearance | Powder |
| Formula | C13H19NO3 | M.Wt | 237.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
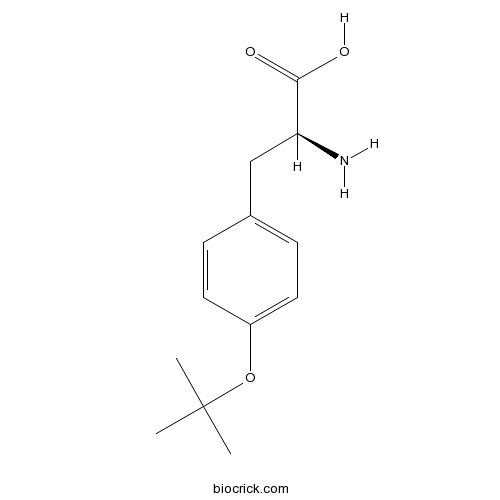
| Chemical Name | (2S)-2-amino-3-[4-[(2-methylpropan-2-yl)oxy]phenyl]propanoic acid | ||
| SMILES | CC(C)(C)OC1=CC=C(C=C1)CC(C(=O)O)N | ||
| Standard InChIKey | SNZIFNXFAFKRKT-NSHDSACASA-N | ||
| Standard InChI | InChI=1S/C13H19NO3/c1-13(2,3)17-10-6-4-9(5-7-10)8-11(14)12(15)16/h4-7,11H,8,14H2,1-3H3,(H,15,16)/t11-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

H-Tyr(tBu)-OH Dilution Calculator

H-Tyr(tBu)-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.2141 mL | 21.0704 mL | 42.1408 mL | 84.2815 mL | 105.3519 mL |
| 5 mM | 0.8428 mL | 4.2141 mL | 8.4282 mL | 16.8563 mL | 21.0704 mL |
| 10 mM | 0.4214 mL | 2.107 mL | 4.2141 mL | 8.4282 mL | 10.5352 mL |
| 50 mM | 0.0843 mL | 0.4214 mL | 0.8428 mL | 1.6856 mL | 2.107 mL |
| 100 mM | 0.0421 mL | 0.2107 mL | 0.4214 mL | 0.8428 mL | 1.0535 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
H-Tyr(tBu)-OH
- H-Ser(tBu)-OH
Catalog No.:BCC3032
CAS No.:18822-58-7
- NocII
Catalog No.:BCC5704
CAS No.:188119-47-3
- AWD 131-138
Catalog No.:BCC4045
CAS No.:188116-07-6
- Odoroside H
Catalog No.:BCN1163
CAS No.:18810-25-8
- Abacavir sulfate
Catalog No.:BCC5023
CAS No.:188062-50-2
- Bikinin
Catalog No.:BCC5582
CAS No.:188011-69-0
- Boc-Glu-NH2
Catalog No.:BCC3387
CAS No.:18800-74-3
- 5-Acetyltaxachitriene A
Catalog No.:BCN7412
CAS No.:187988-48-3
- Trp-Lys-Tyr-Met-Val-Met
Catalog No.:BCC5816
CAS No.:187986-17-0
- WKYMVM trifluoroacetate salt
Catalog No.:BCC5815
CAS No.:187986-11-4
- Acetylcorynoline
Catalog No.:BCN1239
CAS No.:18797-80-3
- (+)-Corynoline
Catalog No.:BCN1235
CAS No.:18797-79-0
- (±)-Octanoylcarnitine chloride
Catalog No.:BCC6715
CAS No.:18822-86-1
- Methylproamine
Catalog No.:BCC1741
CAS No.:188247-01-0
- (±)-Propionylcarnitine chloride
Catalog No.:BCC6719
CAS No.:18828-58-5
- 8alpha-(2-Methylacryloyloxy)hirsutinolide
Catalog No.:BCN7109
CAS No.:188293-70-1
- Californidine
Catalog No.:BCC8137
CAS No.:18830-99-4
- Massonianoside B
Catalog No.:BCN1164
CAS No.:188300-19-8
- Isodomoic acid G
Catalog No.:BCN1839
CAS No.:188346-81-8
- Pellitorine
Catalog No.:BCN4043
CAS No.:18836-52-7
- MAFP
Catalog No.:BCC7059
CAS No.:188404-10-6
- Paederosidic acid
Catalog No.:BCN3438
CAS No.:18842-98-3
- Scandoside
Catalog No.:BCN3449
CAS No.:18842-99-4
- SBI-0206965
Catalog No.:BCC3984
CAS No.:1884220-36-3
A structure-activity relationship study and combinatorial synthetic approach of C-terminal modified bifunctional peptides that are delta/mu opioid receptor agonists and neurokinin 1 receptor antagonists.[Pubmed:18266313]
J Med Chem. 2008 Mar 13;51(5):1369-76.
A series of bifunctional peptides with opioid agonist and substance P antagonist bioactivities were designed with the concept of overlapping pharmacophores. In this concept, the bifunctional peptides were expected to interact with each receptor separately in the spinal dorsal horn where both the opioid receptors and the NK1 receptors were found to be expressed, to show an enhanced analgesic effect, no opioid-induced tolerance, and to provide better compliance than coadministration of two drugs. Compounds were synthesized using a two-step combinatorial method for C-terminal modification. In the method, the protected C-terminal-free carboxyl peptide, Boc-Tyr( tBu)- d-Ala-Gly Phe-Pro-Leu-Trp(Boc)-OH, was synthesized as a shared intermediate using Fmoc solid phase chemistry on a 2-chlorotrityl resin. This intermediate was esterified or amidated in solution phase. The structure-activity relationships (SAR) showed that the C-terminus acted as not only a critical pharmacophore for the substance P antagonist activities, but as an address region for the opioid agonist pharmacophore that is structurally distant from the C-terminal. Among the peptides, H-Tyr- d -Ala-Gly-Phe-Pro-Leu-Trp-NH-Bzl ( 3) demonstrated high binding affinities at both delta and mu receptors ( K i = 10 and 0.65 nM, respectively) with efficient agonist functional activity in the mouse isolated vas deferens (MVD) and guinea pig isolated ileum (GPI) assays (IC 50 = 50 and 13 nM, respectively). Compound 3 also showed a good antagonist activity in the GPI assay with substance P stimulation ( K e = 26 nM) and good affinity for the hNK1 receptor ( K i = 14 nM). Consequently, compound 3 is expected to be a promising and novel type of analgesic with bifunctional activities.
Cyclic morphiceptin analogs: cyclization studies and opioid activities in vitro.[Pubmed:8985782]
Int J Pept Protein Res. 1996 Dec;48(6):495-502.
Attempts were undertaken to develop cyclic beta-casomorphin-5 analogs with improved opioid activity profiles by deletion of the glycine residue in position 5, leading to analogs structurally related to the opioid peptide morphiceptin (H-Tyr-Pro-Phe-Pro-NH2). The tetrapeptide sequence Boc-Tyr(tBu)-D-Xaa-Phe-Yaa-OH (Xaa = Lys, Orn, A2bu; Yaa = Pro in L- or D-configuration) was used to study the influence of ring size and chirality on the yield of cyclization between the omega-amino group of Xaa and the C-terminal carboxyl group. In all cases the cyclization reaction was performed under identical experimental conditions to allow a direct comparison with regard to yield and homogeneity. The reaction products were purified by crystallization and liquid chromatography, and were characterized by HPLC, TLC, electrospray mass spectrometry and 1H-NMR spectroscopy. In none of the reactions performed with the cyclization precursors containing proline in the L-configuration could a cyclic monomer be detected, and the cyclodimer (7-9) was the exclusive product in each case. Cyclodimerization was also the favored reaction in the attempted formation of the 11-membered ring of the cyclic [D-A2bu2, D-Pro4]-morphiceptin analog 12, since only traces of the monomer were found. In the case of both the [D-Lys2, D-Pro4]-analog 10 and the [D-Orn2, D-Pro4]-analog 11, the cyclomonomer/cyclodimer ratio was about 80:20. The cyclic monomers 10 and 11 showed high opioid activity in the mu-receptor-representative guinea pig ileum assay (IC50 = 2-5 nM) and in the delta-receptor representative mouse vas deferens assay (IC50 = 50-60 nM), whereas the potency of the cyclodimers was 2-3 orders of magnitude lower in both in vitro bioassays.
Anchor-linked intermediates in peptide amide synthesis are caused by dimeric anchors on the solid supports.[Pubmed:9222996]
J Pept Sci. 1995 May-Jun;1(3):191-200.
Cleavage and kinetic studies have been carried out using commercially obtained H-Tyr(tBu)-5-(4'-aminomethyl-3',5'-dimethoxyphenoxy)valeric acid-TentaGelS (H-Tyr(tBu)-4-ADPV-TentaGelS) and H-Tyr (tBu)-4-ADPV-Ala-aminomethyl-resin (H-Tyr(tBu)-4-ADPV-AM-resin) prepared from commercially available resin and loaded with commercially available Fmoc-4-ADPV-OH amide anchor. Cleavage with pure trifluoroacetic acid (TFA) gave the intermediate H-Tyr-4-ADPV-NH2, which was then degraded to H-Tyr-NH2, and cleavage with TFA/dichloromethane (1:9) yielded H-Tyr-4-ADPV-NH2 which could be isolated in preparative amounts. Cleavage reactions with 15N-labelled H-Ala-4-ADPV-(15N)-Gly-AM-resin yielded the intermediate H-Ala-4-ADPV-NH2, which contained no 15N as demonstrated by 1H-NMR. The analysis of the commercial Fmoc-4-ADPV-OH amide anchor showed the presence of Fmoc-4-ADPV-4-ADPV-OH as an impurity in high amounts. This dimeric anchor molecule is the cause of formation of the anchor-linked peptide intermediate obtained during the cleavage from the resin. The particularly high acid-lability of the amide bond between the two ADPV moieties was utilized to synthesize sidechain and C-terminally 4-ADPV protected pentagastrin on a double-anchor resin, and to cleave it using 5% trifluoroacetic acid in dichloromethane. This method may offer a new way for the synthesis of protected peptide amides with improved solubility to be used in fragment condensation.


