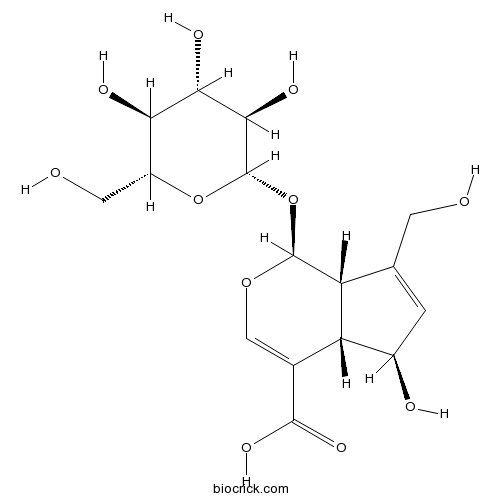
ScandosideCAS# 18842-99-4 |
- Deacetylasperulosidic acid
Catalog No.:BCN3323
CAS No.:14259-55-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 18842-99-4 | SDF | Download SDF |
| PubChem ID | 21602023 | Appearance | Powder |
| Formula | C16H22O11 | M.Wt | 390.3 |
| Type of Compound | Iridoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1S,4aS,5R,7aS)-5-hydroxy-7-(hydroxymethyl)-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-1,4a,5,7a-tetrahydrocyclopenta[c]pyran-4-carboxylic acid | ||
| SMILES | C1=C(C2C(C1O)C(=COC2OC3C(C(C(C(O3)CO)O)O)O)C(=O)O)CO | ||
| Standard InChIKey | ZVXWFPTVHBWJOU-AWQYILTISA-N | ||
| Standard InChI | InChI=1S/C16H22O11/c17-2-5-1-7(19)10-6(14(23)24)4-25-15(9(5)10)27-16-13(22)12(21)11(20)8(3-18)26-16/h1,4,7-13,15-22H,2-3H2,(H,23,24)/t7-,8-,9-,10+,11-,12+,13-,15+,16+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Scandoside exerts anti-inflammatory effect via suppressing NF-κB and MAPK signaling pathways in LPS-induced RAW 264.7 macrophages.It also inhibits LDL-oxidation. |
| Targets | MAPK | NF-κB |
| In vitro | Iridoid glycosides isolated from Oldenlandia diffusa inhibit LDL-oxidation.[Reference: WebLink]Archives of Pharmacal Research, 2005, 28(10):1156-1160.
Iridoid glucosides with insecticidal activity from Galium melanantherum.[Reference: WebLink]Zeitschrift für Naturforschung C, 2007, 62(7-8):597---602.The insecticidal activity of the endemic species Galium melanantherum was evaluated against Crematogaster scutellaris ants and Kalotermes flavicollis termites.
|
| Kinase Assay | Scandoside Exerts Anti-Inflammatory Effect Via Suppressing NF-κB and MAPK Signaling Pathways in LPS-Induced RAW 264.7 Macrophages.[Reference: WebLink]International Journal of Molecular Sciences, 2018, 19(2):457.The iridoids of Hedyotis diffusa Willd play an important role in the anti-inflammatory process, but the specific iridoid with anti-inflammatory effect and its mechanism has not be thoroughly studied.
|

Scandoside Dilution Calculator

Scandoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5621 mL | 12.8107 mL | 25.6213 mL | 51.2426 mL | 64.0533 mL |
| 5 mM | 0.5124 mL | 2.5621 mL | 5.1243 mL | 10.2485 mL | 12.8107 mL |
| 10 mM | 0.2562 mL | 1.2811 mL | 2.5621 mL | 5.1243 mL | 6.4053 mL |
| 50 mM | 0.0512 mL | 0.2562 mL | 0.5124 mL | 1.0249 mL | 1.2811 mL |
| 100 mM | 0.0256 mL | 0.1281 mL | 0.2562 mL | 0.5124 mL | 0.6405 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Paederosidic acid
Catalog No.:BCN3438
CAS No.:18842-98-3
- MAFP
Catalog No.:BCC7059
CAS No.:188404-10-6
- Pellitorine
Catalog No.:BCN4043
CAS No.:18836-52-7
- Isodomoic acid G
Catalog No.:BCN1839
CAS No.:188346-81-8
- Massonianoside B
Catalog No.:BCN1164
CAS No.:188300-19-8
- Californidine
Catalog No.:BCC8137
CAS No.:18830-99-4
- 8alpha-(2-Methylacryloyloxy)hirsutinolide
Catalog No.:BCN7109
CAS No.:188293-70-1
- (±)-Propionylcarnitine chloride
Catalog No.:BCC6719
CAS No.:18828-58-5
- Methylproamine
Catalog No.:BCC1741
CAS No.:188247-01-0
- (±)-Octanoylcarnitine chloride
Catalog No.:BCC6715
CAS No.:18822-86-1
- H-Tyr(tBu)-OH
Catalog No.:BCC3129
CAS No.:18822-59-8
- H-Ser(tBu)-OH
Catalog No.:BCC3032
CAS No.:18822-58-7
- SBI-0206965
Catalog No.:BCC3984
CAS No.:1884220-36-3
- TFM-4AS-1
Catalog No.:BCC6069
CAS No.:188589-61-9
- Cl-4AS-1
Catalog No.:BCC7780
CAS No.:188589-66-4
- GSK3787
Catalog No.:BCC2263
CAS No.:188591-46-0
- 3-hydroxymorindone
Catalog No.:BCN3126
CAS No.:80368-74-7
- 4,5-Di-O-caffeoylquinic acid methyl ester
Catalog No.:BCN6492
CAS No.:188742-80-5
- N-Acetylcaprolactam
Catalog No.:BCC9081
CAS No.:1888-91-1
- SC 560
Catalog No.:BCC7111
CAS No.:188817-13-2
- 8-Glucosyl-5,7-dihydroxy-2-(1-methylpropyl)chromone
Catalog No.:BCN7505
CAS No.:188818-27-1
- Streptozotocin
Catalog No.:BCN3834
CAS No.:18883-66-4
- HX 531
Catalog No.:BCC6082
CAS No.:188844-34-0
- HX 630
Catalog No.:BCC6083
CAS No.:188844-52-2
Estimation of p-coumaric acid as metabolite of E-6-O-p-coumaroyl scandoside methyl ester in rat plasma by HPLC and its application to a pharmacokinetic study.[Pubmed:16388996]
J Chromatogr B Analyt Technol Biomed Life Sci. 2006 Feb 2;831(1-2):303-6.
A rapid and simple high-performance liquid chromatographic (HPLC) method has been developed for the determination of p-coumaric acid in rat plasma and applied to a pharmacokinetic study in rats after administration of a prodrug, E-6-O-p-coumaroyl Scandoside methyl ester, isolated from Hedyotis diffusa (Willd.). Sample preparation involved protein precipitation with acetonitrile. The supernatant was then injected onto a Diamonsil C(18) column (250 mm x 4.6mm i.d., 5 microm). The mobile phase consisted of acetonitrile-water (21:79, v/v) with 1% glacial acetic acid. The UV detector was set at 310 nm. The lower limit of quantification of p-coumaric acid in rat plasma was 0.02 microg/mL. The calibration curves were linear over the concentration range 0.02-5 microg/mL with correlations greater than 0.999. The assay procedure was applied to the study of the metabolite pharmacokinetics of E-6-O-p-coumaroyl Scandoside methyl ester in rat.
[Determination of scandoside methyl ester in Hedyotis chrysotricha by HPLC].[Pubmed:20069906]
Zhongguo Zhong Yao Za Zhi. 2009 Oct;34(20):2619-21.
OBJECTIVE: To develop an HPLC method for determination of Scandoside methyl ester in Hedyotis chrysotricha. METHOD: The determination was carried out on an HC-C18 column elnted with acetonitrile-water (7:93) as mobile phase, and the detection wavelength was set at 238 nm. RESULT: There is a good linearity in the range 22.08-552 mg L(-1) of Scandoside methyl ester and the average recovery is 97.7% (n = 6), RSD 0.72%. CONCLUSION: This method is convenient, quick, and is applicable to the quality control of H. chrysotricha.
[In vivo comparison analysis of scandoside methyl ester metabolites in four kinds of liver microsomes using ultra-performance liquid chromatography combined with electrospray ionization tandem orbitrap mass spectrometry].[Pubmed:25518332]
Yao Xue Xue Bao. 2014 Sep;49(9):1315-9.
In order to clarify the metabolism pathways of Scandoside methyl ester, the analysis of metabolites profiling in four kinds of liver microsomes was performed by using an ultra-performance liquid chromatography/ electrospray-tandem mass spectrometry (UPLC-ESI-MS). The data obtained from the 0 h-incubation and the 2 h-incubation were compared and analyzed. After incubation, 5 metabolites of Scandoside methyl ester were found in rat, Beagles, rhesus monkey and human liver microsome. The results showed that Scandoside methyl ester's major metabolic pathway in the liver microsomes is hydrolysis, oxidation and reduction reactions, and there are certain kinds differences between species. The study provides a research base for further research about iridoid compounds in vivo metabolic pathways.


