3-hydroxymorindoneCAS# 80368-74-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 80368-74-7 | SDF | Download SDF |
| PubChem ID | 86012754 | Appearance | Powder |
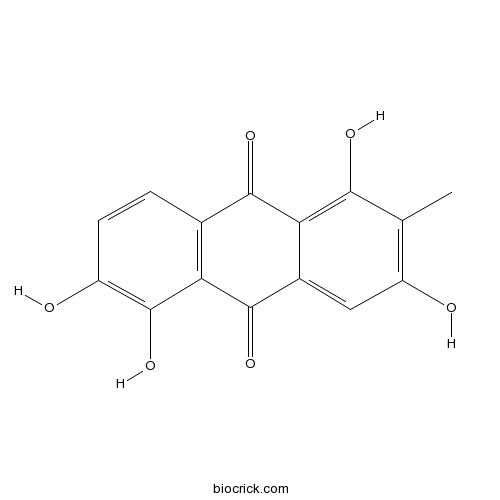
| Formula | C15H10O6 | M.Wt | 286.24 |
| Type of Compound | Anthraquinones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1,3,5,6-tetrahydroxy-2-methylanthracene-9,10-dione | ||
| SMILES | CC1=C(C=C2C(=C1O)C(=O)C3=C(C2=O)C(=C(C=C3)O)O)O | ||
| Standard InChIKey | JCZGUFVZKUDRMM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H10O6/c1-5-9(17)4-7-11(12(5)18)13(19)6-2-3-8(16)15(21)10(6)14(7)20/h2-4,16-18,21H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Methyltanshinonate shows antiplasmodial and antitrypanosomal activities. It may have antioxidative activity. |
| In vitro | Antiplasmodial and antitrypanosomal activity of tanshinone-type diterpenoids from Salvia miltiorrhiza.[Reference: WebLink]Planta Medica, 2011, 77(14):1594-1596.
In a medium throughput screen of 880 plant and fungal extracts for antiprotozoal activity, a dichloromethane extract of Salvia miltiorrhiza roots was active against both Trypanosoma brucei rhodesiense and Plasmodium falciparum.
|
| Structure Identification | Phytochemistry Letters, 2016:S1874390016303500.Determination of phenolic compounds and diterpenes in roots of Salvia miltiorrhiza and Salvia przewalskii by two LC–MS tools: Multi-stage and high resolution tandem mass spectrometry with assessment of antioxidant capacity.[Reference: WebLink]
|

3-hydroxymorindone Dilution Calculator

3-hydroxymorindone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4936 mL | 17.4679 mL | 34.9357 mL | 69.8714 mL | 87.3393 mL |
| 5 mM | 0.6987 mL | 3.4936 mL | 6.9871 mL | 13.9743 mL | 17.4679 mL |
| 10 mM | 0.3494 mL | 1.7468 mL | 3.4936 mL | 6.9871 mL | 8.7339 mL |
| 50 mM | 0.0699 mL | 0.3494 mL | 0.6987 mL | 1.3974 mL | 1.7468 mL |
| 100 mM | 0.0349 mL | 0.1747 mL | 0.3494 mL | 0.6987 mL | 0.8734 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- GSK3787
Catalog No.:BCC2263
CAS No.:188591-46-0
- Cl-4AS-1
Catalog No.:BCC7780
CAS No.:188589-66-4
- TFM-4AS-1
Catalog No.:BCC6069
CAS No.:188589-61-9
- SBI-0206965
Catalog No.:BCC3984
CAS No.:1884220-36-3
- Scandoside
Catalog No.:BCN3449
CAS No.:18842-99-4
- Paederosidic acid
Catalog No.:BCN3438
CAS No.:18842-98-3
- MAFP
Catalog No.:BCC7059
CAS No.:188404-10-6
- Pellitorine
Catalog No.:BCN4043
CAS No.:18836-52-7
- Isodomoic acid G
Catalog No.:BCN1839
CAS No.:188346-81-8
- Massonianoside B
Catalog No.:BCN1164
CAS No.:188300-19-8
- Californidine
Catalog No.:BCC8137
CAS No.:18830-99-4
- 8alpha-(2-Methylacryloyloxy)hirsutinolide
Catalog No.:BCN7109
CAS No.:188293-70-1
- 4,5-Di-O-caffeoylquinic acid methyl ester
Catalog No.:BCN6492
CAS No.:188742-80-5
- N-Acetylcaprolactam
Catalog No.:BCC9081
CAS No.:1888-91-1
- SC 560
Catalog No.:BCC7111
CAS No.:188817-13-2
- 8-Glucosyl-5,7-dihydroxy-2-(1-methylpropyl)chromone
Catalog No.:BCN7505
CAS No.:188818-27-1
- Streptozotocin
Catalog No.:BCN3834
CAS No.:18883-66-4
- HX 531
Catalog No.:BCC6082
CAS No.:188844-34-0
- HX 630
Catalog No.:BCC6083
CAS No.:188844-52-2
- DMA
Catalog No.:BCC1532
CAS No.:188860-26-6
- Hydroxytanshinone IIA
Catalog No.:BCN2497
CAS No.:18887-18-8
- Methyl tanshinonate
Catalog No.:BCN2553
CAS No.:18887-19-9
- Junipediol B 8-O-glucoside
Catalog No.:BCN4022
CAS No.:188894-19-1
- L 760735
Catalog No.:BCC7840
CAS No.:188923-01-5
[Anthraquinones from the roots of Knoxia valerianoides].[Pubmed:22308688]
Zhongguo Zhong Yao Za Zhi. 2011 Nov;36(21):2980-6.
OBJECTIVE: To investigate the chemical constituents of the roots of Knoxia valerianoides and their biological activities. METHOD: The anthraquinones were isolated by using a combination of various chromatographic techniques including column chromatography over silica gel, Sephadex LH-20, and reversed-phase HPLC. Structures of the isolates were identified by their physical-chemical properties and spectroscopic analysis including 2D NMR and MS. Antioxidant, anti-HIV, neuroprotective, and cytotoxic activities were screened by using cell-based models. RESULT: Twenty-two constituents were isolated from an ethanolic extract of the roots of K. valerianoides. Their structures were identified as nordamnacanthal (1), ibericin (2), rubiadin (3), damnacanthol (4), 2-ethoxymethylknoxiavaledin (5), 3-hydroxymorindone (6), knoxiadin (7), 2-formyl knoxiavaledin (8), lucidin (9), xanthopurpurin (10), 1, 3-dihydroxy-2-methoxy-9, 10- anthraquinone (11), lucidin(-methyl ether (12), digiferruginol (13), 3-hydroxy-2-methyl-9,10-anthraquinone (14), rubiadin-1-methyl ether (15), 6-methoxylucidin (-ethyl ether (16), 1,3,6-trihydroxy-2-methyl-9,10-anthraquinone (17), 1,3-dihydroxy-2-hydroxy methyl-6-methoxy-9,10-anthraquinone (18), 1,3,6-trihydroxy-2-methoxymethyl-9,10- anthraquinone (19), 3,6-dihydroxy-2- hydroxymethyl-9,10-anthraquinone (20), and 1,6-dihydroxy-2-methyl-9,10-anthra quinone (21). In the in vitro assays, at a concentration of 1 x 10(-5) mol x L(-1), no compounds were active against human cancer cell lines (HCT-8, Bel7402, BGC-823, A549, and A2780), deserum and glutamate induced PC12-syn cell damage, LPS induced NO production in macrophage, Fe2+-cystine induced rat liver microsomal lipid peroxidation, HIV-1 replication, and protein tyrosine phosphatase 1B (PTP1B). CONCLUSION: Compounds 9-21 were obtained from the roots of K. valerianoides for the first time.


