Hydroxytanshinone IIACAS# 18887-18-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 18887-18-8 | SDF | Download SDF |
| PubChem ID | 5318349 | Appearance | Red Powder |
| Formula | C19H18O4 | M.Wt | 310.35 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 9-hydroxy-1,6,6-trimethyl-8,9-dihydro-7H-naphtho[1,2-g][1]benzofuran-10,11-dione | ||
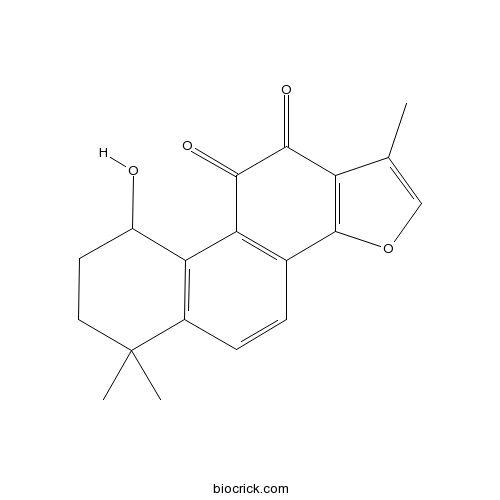
| SMILES | CC1=COC2=C1C(=O)C(=O)C3=C2C=CC4=C3C(CCC4(C)C)O | ||
| Standard InChIKey | UPCWCCRFMPIOAP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H18O4/c1-9-8-23-18-10-4-5-11-15(12(20)6-7-19(11,2)3)14(10)17(22)16(21)13(9)18/h4-5,8,12,20H,6-7H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Hydroxytanshinone ⅡA has good antiproliferative effect on SGC-7901,HeLa, and HepG 2 cell, the values of IC50 are 4.18, 6.08 and10.20 uM, respectively; it has tumor cell proliferation inhibition significantly stronger than the tanshinoneⅡA . |

Hydroxytanshinone IIA Dilution Calculator

Hydroxytanshinone IIA Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2222 mL | 16.1108 mL | 32.2217 mL | 64.4434 mL | 80.5542 mL |
| 5 mM | 0.6444 mL | 3.2222 mL | 6.4443 mL | 12.8887 mL | 16.1108 mL |
| 10 mM | 0.3222 mL | 1.6111 mL | 3.2222 mL | 6.4443 mL | 8.0554 mL |
| 50 mM | 0.0644 mL | 0.3222 mL | 0.6444 mL | 1.2889 mL | 1.6111 mL |
| 100 mM | 0.0322 mL | 0.1611 mL | 0.3222 mL | 0.6444 mL | 0.8055 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- DMA
Catalog No.:BCC1532
CAS No.:188860-26-6
- HX 630
Catalog No.:BCC6083
CAS No.:188844-52-2
- HX 531
Catalog No.:BCC6082
CAS No.:188844-34-0
- Streptozotocin
Catalog No.:BCN3834
CAS No.:18883-66-4
- 8-Glucosyl-5,7-dihydroxy-2-(1-methylpropyl)chromone
Catalog No.:BCN7505
CAS No.:188818-27-1
- SC 560
Catalog No.:BCC7111
CAS No.:188817-13-2
- N-Acetylcaprolactam
Catalog No.:BCC9081
CAS No.:1888-91-1
- 4,5-Di-O-caffeoylquinic acid methyl ester
Catalog No.:BCN6492
CAS No.:188742-80-5
- 3-hydroxymorindone
Catalog No.:BCN3126
CAS No.:80368-74-7
- GSK3787
Catalog No.:BCC2263
CAS No.:188591-46-0
- Cl-4AS-1
Catalog No.:BCC7780
CAS No.:188589-66-4
- TFM-4AS-1
Catalog No.:BCC6069
CAS No.:188589-61-9
- Methyl tanshinonate
Catalog No.:BCN2553
CAS No.:18887-19-9
- Junipediol B 8-O-glucoside
Catalog No.:BCN4022
CAS No.:188894-19-1
- L 760735
Catalog No.:BCC7840
CAS No.:188923-01-5
- Cilengitide
Catalog No.:BCC3942
CAS No.:188968-51-6
- Melilotigenin C
Catalog No.:BCN1165
CAS No.:188970-21-0
- 1-(4-Hydroxy-2,2-dimethylchroman-6-yl)ethanone
Catalog No.:BCN7710
CAS No.:1890153-71-5
- Corynantheine
Catalog No.:BCN3746
CAS No.:18904-54-6
- NGB 2904
Catalog No.:BCC7435
CAS No.:189061-11-8
- [Ala92]-p16 (84-103)
Catalog No.:BCC5837
CAS No.:189064-08-2
- Oroselol
Catalog No.:BCN3907
CAS No.:1891-25-4
- Hulupinic acid
Catalog No.:BCN8019
CAS No.:1891-42-5
- Salmefamol
Catalog No.:BCC1919
CAS No.:18910-65-1
Simultaneous determination of tanshinone IIA and its three hydroxylated metabolites by liquid chromatography/tandem mass spectrometry.[Pubmed:16470728]
Rapid Commun Mass Spectrom. 2006;20(5):815-22.
A rapid and sensitive method based on liquid chromatography/tandem mass spectrometry (LC/MS/MS) for the simultaneous determination of tanshinone IIA and its three hydroxylated metabolites, tanshinone IIB, Hydroxytanshinone IIA and przewaquinone A, in a rat liver microsome was developed and fully validated. A single step of liquid-liquid extraction with ethyl acetate was utilized in this method. Chromatographic separation of the sample matrix from the analytes and the internal standard diazepam was performed using a Shim-pack VP-ODS analytical column. Detection was performed on a triple quadrupole tandem mass spectrometer equipped with an electrospray ionization source and operated in selected reaction monitoring (SRM) mode. The method was linear in the concentration range of 1-500 ng/mL for all analytes. The intra- and inter-day precisions (RSD %) were within 15% and deviations of the assay accuracies were within 15.0% for all analytes. The analytes proved to be stable during sample storage, preparation and analyses. This validated method was successfully applied to the enzyme kinetic study of tanshinone IIA in liver microsome. The elimination of tanshinone IIA and formation of tanshinone IIB and Hydroxytanshinone IIA in the liver microsome all exhibited a sigmoidal kinetics profile. The formation of przewaquinone A shows a typical hyperbolic profile. In addition, this method has now been applied in the analysis of other bio-samples including plasma, urine, bile and feces.
Characterization of metabolites of tanshinone IIA in rats by liquid chromatography/tandem mass spectrometry.[Pubmed:16598708]
J Mass Spectrom. 2006 May;41(5):670-84.
The metabolism of tanshinone IIA was studied in rats after a single-dose intravenous administration. In the present study, 12 metabolites of tanshinone IIA were identified in rat bile, urine and feces with two LC gradients using LC-MS/MS. Seven phase I metabolites and five phase II metabolites of tanshinone IIA were characterized and their molecular structures proposed on the basis of the characteristics of their precursor ions, product ions and chromatographic retention time. The seven phase I metabolites were formed, through two main metabolic routes, which were hydroxylation and dehydrogenation metabolism. M1, M4, M5 and M6 were supposedly tanshinone IIB, Hydroxytanshinone IIA, przewaquinone A and dehydrotanshinone IIA, respectively, by comparing their HPLC retention times and mass spectral patterns with those of the standard compounds. The five phase II metabolites identified in this research were all glucuronide conjugates, all of which showed a neutral loss of 176 Da. M9 and M12 were more abundant than other identified metabolites in the bile, which was the main excretion path of tanshinone IIA and the metabolites. M12 was the main metabolite of tanshinone IIA. M9 and M12 were proposed to be the glucuronide conjugates of two different semiquinones and these semiquinones were the hydrogenation products of dehydrotanshinone IIA and tanshinone IIA, respectively. This hydrogenized reaction may be catalyzed by the NAD(P)H: quinone acceptor oxidoreductase (NQO). The biotransformation pathways of tanshinone IIA were proposed on the basis of this research.


