LonafarnibFtase inhibitor,potent and selective CAS# 193275-84-2 |
- PF-04620110
Catalog No.:BCC2335
CAS No.:1109276-89-2
- Tolcapone
Catalog No.:BCC2334
CAS No.:134308-13-7
- Tipifarnib (Zarnestra)
Catalog No.:BCC2253
CAS No.:192185-72-1
- FK866 (APO866)
Catalog No.:BCC2332
CAS No.:658084-64-1
- A922500
Catalog No.:BCC2333
CAS No.:959122-11-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 193275-84-2 | SDF | Download SDF |
| PubChem ID | 148195 | Appearance | Powder |
| Formula | C27H31Br2ClN4O2 | M.Wt | 638.82 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Sch66336 | ||
| Solubility | DMSO : ≥ 100 mg/mL (156.54 mM) *"≥" means soluble, but saturation unknown. | ||
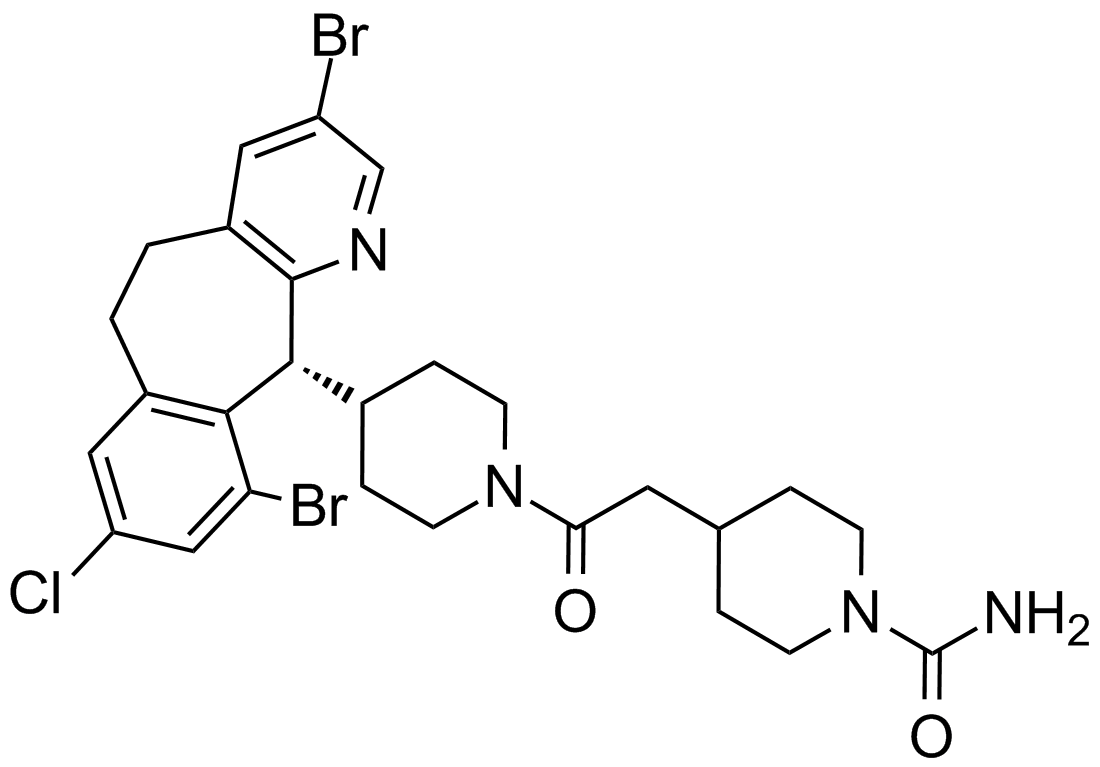
| Chemical Name | 4-[2-[4-[(11R)-3,10-dibromo-8-chloro-6,11-dihydro-5H-benzo[1,2]cyclohepta[2,4-b]pyridin-11-yl]piperidin-1-yl]-2-oxoethyl]piperidine-1-carboxamide | ||
| SMILES | C1CN(CCC1CC(=O)N2CCC(CC2)C3C4=C(C=C(C=C4CCC5=CC(=CN=C35)Br)Cl)Br)C(=O)N | ||
| Standard InChIKey | DHMTURDWPRKSOA-RUZDIDTESA-N | ||
| Standard InChI | InChI=1S/C27H31Br2ClN4O2/c28-20-12-19-2-1-18-13-21(30)14-22(29)24(18)25(26(19)32-15-20)17-5-9-33(10-6-17)23(35)11-16-3-7-34(8-4-16)27(31)36/h12-17,25H,1-11H2,(H2,31,36)/t25-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Lonafarnib is an inhibitor of farnesyl transferase (FT) with IC50 values of 1.9, 2.8, 5.2 and 10-100 nM for H-Ras, N-Ras, K-Ras and Rheb, respectively. | ||||||
| Targets | FT | FT | FT | FT | |||
| IC50 | 1.9 nM (H-Ras) | 2.8 nM (N-Ras) | 5.2 nM (K-Ras) | 10-100 nM (Rheb) | |||

Lonafarnib Dilution Calculator

Lonafarnib Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5654 mL | 7.8269 mL | 15.6539 mL | 31.3077 mL | 39.1347 mL |
| 5 mM | 0.3131 mL | 1.5654 mL | 3.1308 mL | 6.2615 mL | 7.8269 mL |
| 10 mM | 0.1565 mL | 0.7827 mL | 1.5654 mL | 3.1308 mL | 3.9135 mL |
| 50 mM | 0.0313 mL | 0.1565 mL | 0.3131 mL | 0.6262 mL | 0.7827 mL |
| 100 mM | 0.0157 mL | 0.0783 mL | 0.1565 mL | 0.3131 mL | 0.3913 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Lonafarnib (SCH66336, Sarasar) is an potent, selective, orally, bioavailable tricyclic nonpeptidyl nonsulfhydry inhibitor of farnesyltransferase (FTase).[1] It is a small molecular with the formula of C27H31Br2ClN4O2 and molecular weight of 638.82. Farnesylated Ras proteins was found to regulate signal transduction pathways which drive cell proliferation, growth and survival and be required for its membrane localization.[1, 2] Lonafarnib inhibits the post-translational farnesylcation of ras proteins, therefore blocking translocation of RAS to the plasma membrane.[3]
Reference
[1] Eric W, Malcolm J. M, Kim N. C, D. Scott E, et al. A multinomial Phase II study of lonafarnib (SCH 66336) in patients with refractory urothelial cancer. Urologic Oncology: Seminars and Original Investigations. 2005, 23. 143-149.
[2] Gongjie L, Stacey A. T, Cindy H. M, Yunsheng H, W. Robert B, et al. Continuous and intermittent dosing of lonafarnib potentiates the therapeutic efficacy of docetaxel on preclinical human prostate cancer models. Int. J. Cancer. 2009, 125. 2711–2720.
[3] Vasiliki A. N, Alexander J. S, Keith T. F, Hensin T, et al. Melanoma: New Insights and New Therapies. J Invest Dermatol. 2012, 132. 854–863.
- 1-O-Acetyl-6alpha-O-(2-methylbutyryl)britannilactone
Catalog No.:BCN7747
CAS No.:1932687-71-2
- cis-Moschamine
Catalog No.:BCN3901
CAS No.:193224-24-7
- Otamixaban
Catalog No.:BCC1827
CAS No.:193153-04-7
- Cardamonin
Catalog No.:BCN1184
CAS No.:19309-14-9
- CTS-1027
Catalog No.:BCC1502
CAS No.:193022-04-7
- ZM323881
Catalog No.:BCC2073
CAS No.:193001-14-8
- ZM 323881 HCl
Catalog No.:BCC5098
CAS No.:193000-39-4
- Ximelagatran
Catalog No.:BCC6382
CAS No.:192939-46-1
- LY310762
Catalog No.:BCC5052
CAS No.:192927-92-7
- 2-Amino-2'-nitrodiphenyl sulfide
Catalog No.:BCC8522
CAS No.:19284-81-2
- ES 936
Catalog No.:BCC6102
CAS No.:192820-78-3
- Cyclomulberrin
Catalog No.:BCN3374
CAS No.:19275-51-5
- 5-Aminoindazole
Catalog No.:BCC8734
CAS No.:19335-11-6
- Co 101244 hydrochloride
Catalog No.:BCC7369
CAS No.:193356-17-1
- Tartrazine
Catalog No.:BCN2217
CAS No.:1934-21-0
- Zeylenone
Catalog No.:BCC8268
CAS No.:193410-84-3
- Fmoc-Glu(Edans)-OH
Catalog No.:BCC3491
CAS No.:193475-66-0
- Mulberroside F
Catalog No.:BCN2908
CAS No.:193483-95-3
- IPAG
Catalog No.:BCC5662
CAS No.:193527-91-2
- SB 239063
Catalog No.:BCC1923
CAS No.:193551-21-2
- Calcifediol
Catalog No.:BCC4949
CAS No.:19356-17-3
- GB 1b
Catalog No.:BCN7385
CAS No.:19360-72-6
- Terrestrosin K
Catalog No.:BCN2935
CAS No.:193605-07-1
- SB 216641 hydrochloride
Catalog No.:BCC6987
CAS No.:193611-67-5
The FNTB promoter polymorphism rs11623866 as a potential predictive biomarker for lonafarnib treatment of ovarian cancer patients.[Pubmed:26033044]
Br J Clin Pharmacol. 2015 Nov;80(5):1139-48.
AIM: Despite promising preclinical findings regarding clinical utility of farnesyltransferase inhibitors (FTI), such as Lonafarnib, success of clinical trials is limited. A multicentre AGO-OVAR-15 phase II trial reported an unfavourable effect of Lonafarnib on the outcome of patients with advanced ovarian cancer. This study was performed as a genetic subgroup analysis of the AGO-OVAR-15 trial, and investigated the utility of the promoter polymorphism rs11623866 of the farnesyltransferase ss-subunit gene (FNTB) in predicting the clinical effectiveness of Lonafarnib. METHODS: The influence of rs11623866 (c.-609G > C) on FNTB promoter activity was investigated by electrophoretic-mobility-shift assay, luciferase-reporter assay and RT-qPCR. A total of 57 out of 105 patients from the AGO-OVAR-15 trial, treated with carboplatin and paclitaxel +/- Lonafarnib, was genotyped for rs11623866 by restriction fragment length polymorphism analysis. Genotype-dependent survival analysis was performed by Kaplan-Meier analysis. RESULTS: The presence of the G allele was associated with increased FNTB promoter activity compared with the C allele. An unfavourable effect of Lonafarnib was limited to patients carrying a GG genotype (HRPFS 6.2, 95%CI = 2.01, 19.41, P = 0.002; HROS 9.6, 95%CI = 1.89, 48.54, P = 0.006). Median progression free survival (PFS) for patients with the GG genotype in the Lonafarnib treated arm was 10 months, whereas median PFS without FTI-treatment was 40 months. Median overall survival (OS) in the Lonafarnib-treated group was 19 months, whereas median OS was not reached in the untreated group. CONCLUSIONS: Discrepancies between preclinical success and clinical failure may be due to the patients' genetic variability of FNTB. Therefore, our results may encourage retrospective evaluation of FNTB polymorphisms in previous FTI studies, especially those reporting positive FTI response.
Oral prenylation inhibition with lonafarnib in chronic hepatitis D infection: a proof-of-concept randomised, double-blind, placebo-controlled phase 2A trial.[Pubmed:26189433]
Lancet Infect Dis. 2015 Oct;15(10):1167-1174.
BACKGROUND: Therapies for chronic hepatitis delta virus (HDV) infection are unsatisfactory. Prenylation is essential for HDV and inhibition abrogates HDV production in experimental models. In a proof-of-concept study, we aimed to assess the effect on HDV RNA levels, safety, and tolerability of the prenylation inhibitor Lonafarnib in patients with chronic delta hepatitis. METHODS: In this phase 2A double-blind, randomised, placebo-controlled study, patients aged 18 years or older with chronic HDV infection were randomly assigned (3:1 in group 1 and 2:1 in group 2) to receive Lonafarnib 100 mg (group 1) or Lonafarnib 200 mg (group 2) twice daily for 28 days with 6 months' follow-up. Participants were randomised by random-number tables blocked in groups of four without stratification. Both groups enrolled six treatment participants and two placebo participants. Group 1 placebo patients received open-label Lonafarnib as group 2 participants. The primary therapeutic endpoint was a decrease in HDV RNA viral titre in serum and the primary safety endpoint was the ability to tolerate the drug at the prescribed dose for the full 4-week duration, defined as drug discontinuation due to intolerance or grade 3/4 adverse events. This trial is registered with ClinicalTrials.gov, number NCT01495585. FINDINGS: Between Jan 19, 2012, and April 28, 2014, 14 patients were enrolled, of whom eight were assigned to group 1 and six were assigned to group 2. At day 28, compared with placebo, mean log HDV RNA declines from baseline were -0.73 log IU/mL in group 1 (95% CI 0.17-1.31; p=0.03) and -1.54 log IU/mL in group 2 (1.21-1.93; p<0.0001). Lonafarnib serum concentrations correlated with HDV RNA change (r(2)=0.78, p<0.0001). Model fits show that hepatitis B surface antigen (HBsAg) remained stable after a short pharmacological delay (0.75 days [SE 0.24]), Lonafarnib effectiveness in blocking HDV production was greater in group 2 than in group 1 (0.952 [SE 0.06] vs 0.739 [0.05], p<0.001), and the HDV half-life was 1.62 days (0.07). There was no evidence of virological resistance. Adverse events were mainly mild to moderate with group 1 patients experiencing diarrhoea in three patients (50%) and nausea in two patients (33%) and in group 2 with all patients (100%) experiencing nausea, diarrhoea, abdominal bloating, and weight loss greater than 2 kg (mean of 4 kg). No treatment discontinuations occurred in any treatment groups. INTERPRETATION: Treatment of chronic HDV with Lonafarnib significantly reduces virus levels. The decline in virus levels significantly correlated with serum drug levels, providing further evidence for the efficacy of prenylation inhibition in chronic HDV. FUNDING: National Institute of Diabetes and Digestive and Kidney Diseases and National Cancer Institute, National Institutes of Health, and Eiger Biopharmaceuticals Inc.
Clinical Trial of the Protein Farnesylation Inhibitors Lonafarnib, Pravastatin, and Zoledronic Acid in Children With Hutchinson-Gilford Progeria Syndrome.[Pubmed:27400896]
Circulation. 2016 Jul 12;134(2):114-25.
BACKGROUND: Hutchinson-Gilford progeria syndrome is an extremely rare, fatal, segmental premature aging syndrome caused by a mutation in LMNA yielding the farnesylated aberrant protein progerin. Without progerin-specific treatment, death occurs at an average age of 14.6 years from an accelerated atherosclerosis. A previous single-arm clinical trial demonstrated that the protein farnesyltransferase inhibitor Lonafarnib ameliorates some aspects of cardiovascular and bone disease. This present trial sought to further improve disease by additionally inhibiting progerin prenylation. METHODS: Thirty-seven participants with Hutchinson-Gilford progeria syndrome received pravastatin, zoledronic acid, and Lonafarnib. This combination therapy was evaluated, in addition to descriptive comparisons with the prior Lonafarnib monotherapy trial. RESULTS: No participants withdrew because of side effects. Primary outcome success was predefined by improved per-patient rate of weight gain or carotid artery echodensity; 71.0% of participants succeeded (P<0.0001). Key cardiovascular and skeletal secondary variables were predefined. Secondary improvements included increased areal (P=0.001) and volumetric (P<0.001-0.006) bone mineral density and 1.5- to 1.8-fold increases in radial bone structure (P<0.001). Median carotid artery wall echodensity and carotid-femoral pulse wave velocity demonstrated no significant changes. Percentages of participants with carotid (5% to 50%; P=0.001) and femoral (0% to 12%; P=0.13) artery plaques and extraskeletal calcifications (34.4% to 65.6%; P=0.006) increased. Other than increased bone mineral density, no improvement rates exceeded those of the prior Lonafarnib monotherapy treatment trial. CONCLUSIONS: Comparisons with Lonafarnib monotherapy treatment reveal additional bone mineral density benefit but likely no added cardiovascular benefit with the addition of pravastatin and zoledronic acid. CLINICAL TRIAL REGISTRATION: URL: http://www.clinicaltrials.gov. Unique identifiers: NCT00879034 and NCT00916747.


