Ximelagatranthrombin inhibitor,orally available CAS# 192939-46-1 |
- Bazedoxifene HCl
Catalog No.:BCC4492
CAS No.:198480-56-7
- Ethynodiol diacetate
Catalog No.:BCC4483
CAS No.:297-76-7
- (E)-2-Decenoic acid
Catalog No.:BCC1292
CAS No.:334-49-6
- Estriol
Catalog No.:BCN2235
CAS No.:50-27-1
- Hexestrol
Catalog No.:BCC4484
CAS No.:84-16-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 192939-46-1 | SDF | Download SDF |
| PubChem ID | 9574101 | Appearance | Powder |
| Formula | C24H35N5O5 | M.Wt | 473.57 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 250 mg/mL (527.91 mM; Need ultrasonic) Methanol : 62.5 mg/mL (131.98 mM; Need ultrasonic) | ||
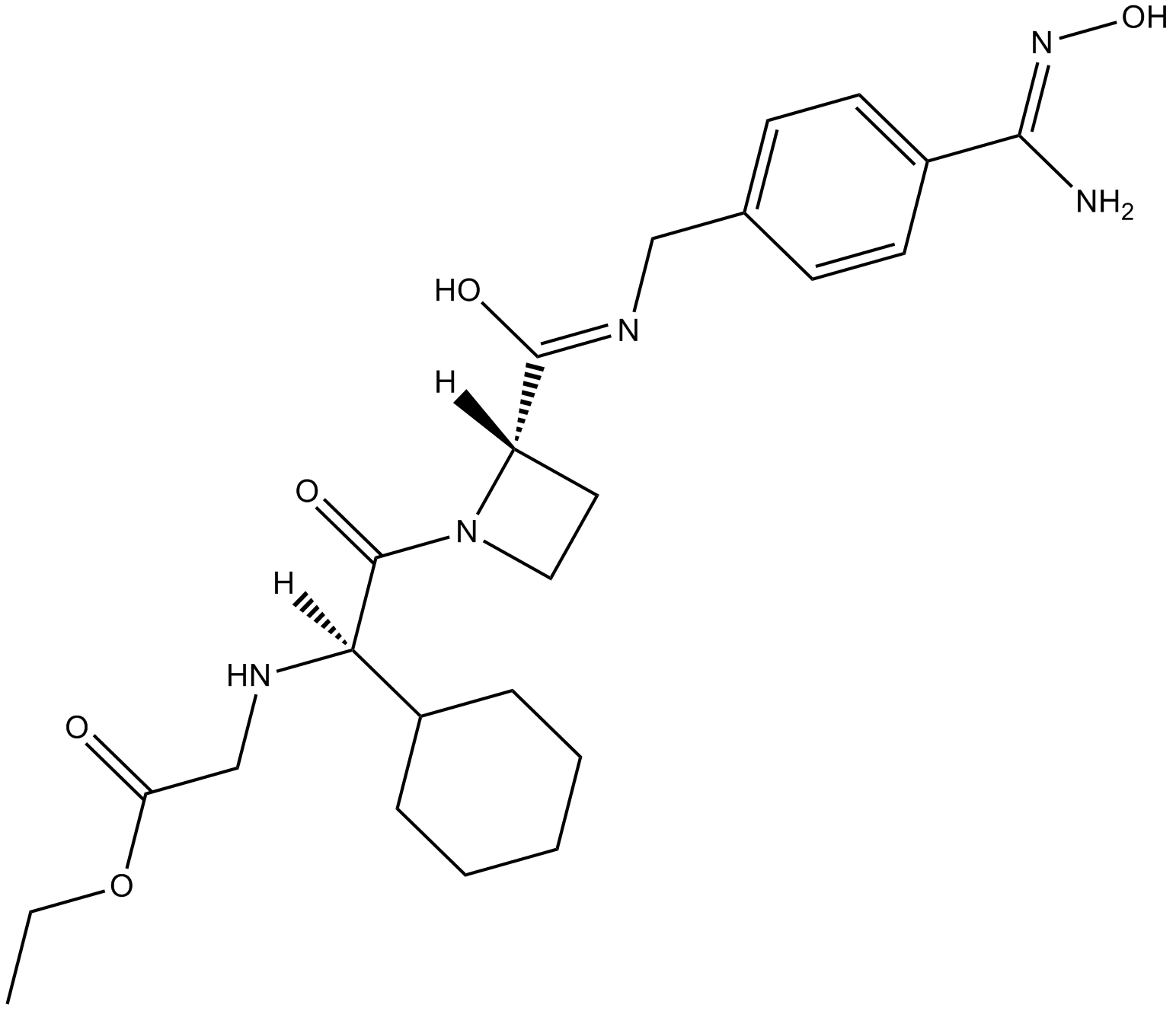
| Chemical Name | ethyl 2-[[(1R)-1-cyclohexyl-2-[(2S)-2-[[4-[(Z)-N'-hydroxycarbamimidoyl]phenyl]methylcarbamoyl]azetidin-1-yl]-2-oxoethyl]amino]acetate | ||
| SMILES | CCOC(=O)CNC(C1CCCCC1)C(=O)N2CCC2C(=O)NCC3=CC=C(C=C3)C(=NO)N | ||
| Standard InChIKey | ZXIBCJHYVWYIKI-PZJWPPBQSA-N | ||
| Standard InChI | InChI=1S/C24H35N5O5/c1-2-34-20(30)15-26-21(17-6-4-3-5-7-17)24(32)29-13-12-19(29)23(31)27-14-16-8-10-18(11-9-16)22(25)28-33/h8-11,17,19,21,26,33H,2-7,12-15H2,1H3,(H2,25,28)(H,27,31)/t19-,21+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ximelagatran Dilution Calculator

Ximelagatran Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ximelagatran is an orally available inhibitor of thrombin [1].
Thrombin is a serine protease that converts soluble fibrinogen into insoluble fibrin, which ultimately results in the reduction of blood loss. Thrombin plays an important role in haemostasis and thrombosis [1].
Ximelagatran is an orally available thrombin inhibitor. In rats with thrombus, oral administration of ximelagatran dose-dependently reduced thrombus size with ID50 value of 15 μmol/kg. Ximelagatran (20 μmol/ kg) reduced thrombus weight to 11.1 ± 1.3 mg, compared to 27.3 ± 2.7 mg in the control group [1]. In healthy male volunteers, oral administration of ximelagatran (60 mg p.o.) significantly reduced the levels of thrombin-antithrombin complex (TAT) and prothrombin fragment 1+2 (F1+2) in shed blood at 2 and 4 h post-dosing, which returned to the normal levels at 10 h post-dosing [2]. In patients with both pulmonary embolism (PE) and deep vein thrombosis (DVT), oral administration of ximelagatran (48 mg twice daily for 6-9 days) increased plasma activated partial thromboplastin time by 2-fold and improved clinical symptoms, including dyspnoea, cough, chest pain, oedema and pain in the affected leg [3].
References:
[1]. Carlsson S, Elg M, Mattsson C. Effects of ximelagatran, the oral form of melagatran, in the treatment of caval vein thrombosis in conscious rats. Thromb Res, 2002, 107(3-4): 163-168.
[2]. Sarich TC, Eriksson UG, Mattsson C, et al. Inhibition of thrombin generation by the oral direct thrombin inhibitor ximelagatran in shed blood from healthy male subjects. Thromb Haemost, 2002, 87(2): 300-305.
[3]. Wåhlander K, Lapidus L, Olsson CG, et al. Pharmacokinetics, pharmacodynamics and clinical effects of the oral direct thrombin inhibitor ximelagatran in acute treatment of patients with pulmonary embolism and deep vein thrombosis. Thromb Res, 2002, 107(3-4): 93-99.
- LY310762
Catalog No.:BCC5052
CAS No.:192927-92-7
- 2-Amino-2'-nitrodiphenyl sulfide
Catalog No.:BCC8522
CAS No.:19284-81-2
- ES 936
Catalog No.:BCC6102
CAS No.:192820-78-3
- Cyclomulberrin
Catalog No.:BCN3374
CAS No.:19275-51-5
- Cudraflavone B
Catalog No.:BCN8067
CAS No.:19275-49-1
- 3-Isomangostin
Catalog No.:BCN1214
CAS No.:19275-46-8
- Lopinavir
Catalog No.:BCC3621
CAS No.:192725-17-0
- C527
Catalog No.:BCC3972
CAS No.:192718-06-2
- PD 161570
Catalog No.:BCC7765
CAS No.:192705-80-9
- H-DL-HoSer-OH
Catalog No.:BCC3242
CAS No.:1927-25-9
- 9-Benzoylcarbazole
Catalog No.:BCC8799
CAS No.:19264-68-7
- PPADS tetrasodium salt
Catalog No.:BCC6725
CAS No.:192575-19-2
- ZM 323881 HCl
Catalog No.:BCC5098
CAS No.:193000-39-4
- ZM323881
Catalog No.:BCC2073
CAS No.:193001-14-8
- CTS-1027
Catalog No.:BCC1502
CAS No.:193022-04-7
- Cardamonin
Catalog No.:BCN1184
CAS No.:19309-14-9
- Otamixaban
Catalog No.:BCC1827
CAS No.:193153-04-7
- cis-Moschamine
Catalog No.:BCN3901
CAS No.:193224-24-7
- 1-O-Acetyl-6alpha-O-(2-methylbutyryl)britannilactone
Catalog No.:BCN7747
CAS No.:1932687-71-2
- Lonafarnib
Catalog No.:BCC2331
CAS No.:193275-84-2
- 5-Aminoindazole
Catalog No.:BCC8734
CAS No.:19335-11-6
- Co 101244 hydrochloride
Catalog No.:BCC7369
CAS No.:193356-17-1
- Tartrazine
Catalog No.:BCN2217
CAS No.:1934-21-0
- Zeylenone
Catalog No.:BCC8268
CAS No.:193410-84-3
Synthesis of ximelagatran, melagatran, hydroxymelagatran, and ethylmelagatran in H-3 labeled form.[Pubmed:24285415]
J Labelled Comp Radiopharm. 2013 May 30;56(6):334-7.
In support of a study designed to better understand the liver toxicity of Ximelagatran, Ximelagatran, and melagatran, hydroxymelagatran and ethylmelagatran were prepared in tritium labeled form. Incorporation of tritium was achieved by hydrogen isotope exchange using Crabtree's catalyst and later with N-heterocyclic containing Ir catalyst. The tritiated product was then converted into the four target compounds to afford them in high purity and specific activity.
In Silico and In Vitro Analysis of Interaction between Ximelagatran and Human Leukocyte Antigen (HLA)-DRB1*07:01.[Pubmed:28338626]
Int J Mol Sci. 2017 Mar 24;18(4). pii: ijms18040694.
Idiosyncratic Ximelagatran-induced hepatotoxicity has been reported to be associated with human leukocyte antigen (HLA)-DRB1*07:01 and Ximelagatran has been reported to inhibit the binding of the ligand peptide to HLA-DRB1*07:01 in vitro. In order to predict the possible interaction modes of Ximelagatran with HLA-DR molecules, in silico docking simulations were performed. Molecular dynamics (MD) simulations were also performed to predict the effect of Ximelagatran on the binding mode of the ligand peptide to HLA-DRB1*07:01. A series of in silico simulations supported the inhibitory effect of Ximelagatran on the binding of the ligand peptide to HLA-DRB1*07:01 in vitro. Furthermore, direct interactions of Ximelagatran with HLA-DR molecules were evaluated in vitro, which supported the simulated interaction mode of Ximelagatran with HLA-DRB1*07:01. These results indicated that Ximelagatran directly interacts with the peptide binding groove of HLA-DRB1*07:01 and competes with the ligand peptide for the binding site, which could alter the immune response and lead to the idiosyncratic Ximelagatran-induced hepatotoxicity.
Expression and Function of mARC: Roles in Lipogenesis and Metabolic Activation of Ximelagatran.[Pubmed:26378779]
PLoS One. 2015 Sep 17;10(9):e0138487.
Recently two novel enzymes were identified in the outer mitochondrial membrane, mARC1 and mARC2. These molybdenum containing enzymes can reduce a variety of N-hydroxylated compounds, such as N-hydroxy-guanidines and sulfohydroxamic acids, as well as convert nitrite into nitric oxide (NO). However, their endogenous functions remain unknown. Here we demonstrate a specific developmental pattern of expression of these enzymes. mARC1, but not mARC2, was found to be expressed in fetal human liver, whereas both, in particular mARC2, are abundant in adult liver and also expressed in omental and subcutaneous fat. Caloric diet restriction of obese patients caused a decreased expression of mARC2 in liver, similar to that seen in the livers of starved rats. Knock down of mARC2 expression by siRNA in murine adipocytes had statistically significant effect on the level of diglycerides and on the fatty acid composition of some triglycerides, concomitantly a clear trend toward the reduced formation of most of triglyceride and phospholipid species was observed. The involvement of mARC2 in the metabolism of the hepatotoxic drug Ximelagatran was evaluated in hepatocytes and adipocytes. Ximelagatran was shown to cause oxidative stress and knock down of mARC2 in adipocytes prevented Ximelagatran induced inhibition of mitochondrial respiration. In conclusion, our data indicate that mARC1 and mARC2 have different developmental expression profiles, and that mARC2 is involved in lipogenesis, is regulated by nutritional status and responsible for activation of Ximelagatran into a mitotoxic metabolite(s).
Predicting potential liver toxicity from phase 2 data: a case study with ximelagatran.[Pubmed:24623062]
Stat Med. 2014 Jul 30;33(17):2914-23.
Ximelagatran was denied marketing approval in the USA and was withdrawn from those markets in which it had been approved, because of concerns over potential liver toxicity. A retrospective analysis of phase 2 data relating to liver toxicity is performed using the methods of extreme value modelling. The analysis reveals that the phase 2 data were predictive of the phase 3 results and, had the methods been available at the time, such analysis would have provided valuable information relating to the decision to proceed with further development of the compound.


