OtamixabanDirect factor Xa inhibitors,potent and selective CAS# 193153-04-7 |
- Bivalirudin Trifluoroacetate
Catalog No.:BCC1421
CAS No.:128270-60-0
- Dabigatran ethyl ester
Catalog No.:BCC1512
CAS No.:429658-95-7
- 5-R-Rivaroxaban
Catalog No.:BCC1313
CAS No.:865479-71-6
- Dabigatran etexilate mesylate
Catalog No.:BCC1511
CAS No.:872728-81-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 193153-04-7 | SDF | Download SDF |
| PubChem ID | 5496659 | Appearance | Powder |
| Formula | C25H26N4O4 | M.Wt | 446.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | FXV673 | ||
| Solubility | DMSO : 50 mg/mL (111.98 mM; Need ultrasonic) | ||
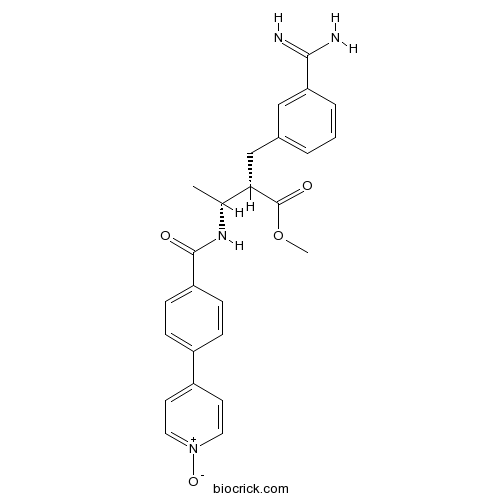
| Chemical Name | methyl (2R,3R)-2-[(3-carbamimidoylphenyl)methyl]-3-[[4-(1-oxidopyridin-1-ium-4-yl)benzoyl]amino]butanoate | ||
| SMILES | CC(C(CC1=CC=CC(=C1)C(=N)N)C(=O)OC)NC(=O)C2=CC=C(C=C2)C3=CC=[N+](C=C3)[O-] | ||
| Standard InChIKey | PFGVNLZDWRZPJW-OPAMFIHVSA-N | ||
| Standard InChI | InChI=1S/C25H26N4O4/c1-16(22(25(31)33-2)15-17-4-3-5-21(14-17)23(26)27)28-24(30)20-8-6-18(7-9-20)19-10-12-29(32)13-11-19/h3-14,16,22H,15H2,1-2H3,(H3,26,27)(H,28,30)/t16-,22-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Otamixaban(FXV673) is a potent, selective, rapid acting, competitive and reversible inhibitor of fXa with a Ki value of 0.5 nM. | |||||
| Targets | Factor Xa | |||||
| IC50 | 0.5 nM (Ki) | |||||

Otamixaban Dilution Calculator

Otamixaban Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2395 mL | 11.1975 mL | 22.3949 mL | 44.7898 mL | 55.9873 mL |
| 5 mM | 0.4479 mL | 2.2395 mL | 4.479 mL | 8.958 mL | 11.1975 mL |
| 10 mM | 0.2239 mL | 1.1197 mL | 2.2395 mL | 4.479 mL | 5.5987 mL |
| 50 mM | 0.0448 mL | 0.2239 mL | 0.4479 mL | 0.8958 mL | 1.1197 mL |
| 100 mM | 0.0224 mL | 0.112 mL | 0.2239 mL | 0.4479 mL | 0.5599 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Otamixaban (FXV673) is a potent, selective, rapid acting, competitive and reversible inhibitor of factor Xa with IC50 value of 0.4 nM.[5]
Factor Xa is a serine endopeptidase which is activated into factor Xa by both factor IX with its cofactor, factor VIII known as intrinsic Xase, and factor VII with its cofactor, tissue factor. Factor Xa (fXa) is a pivotal serine protease situated at the juncture of the intrinsic and extrinsic pathways of the blood coagulation cascade.[2] Its singular role in thrombin activation and potentiating effects on clot formation makes it as a target for therapeutic intervention. FXa is responsible for the initiation of the coagulation cascade,[3] cleaving prothrombin to its active form thrombin, which then acts to convert soluble fibrinogen to insoluble fibrin and to activate platelets. Stabilization of the platelet aggregation by fibrin mesh ultimately leads to clot formation.[4] Thus, the inhibition of fXa (in the physiologically relevant prothrombinase complex) represents an attractive target for the development of novel antithrombotic agents.[5]
Otamixaban (FXV673) selectively and competitively inhibit FXa, suppressing prothrombin activity at the sites of blood clot (thrombus) formation. This leads to a decrease in blood clot formation in a dose dependent manner.[1] Reducing blood clot formation will decrease blood flow blockages, thus possibly lowering the risk of myocardial infarction, unstable angina, venous thrombosis, and ischemic stroke.[6] In conclusion, FXV673 is an effective inhibitor of fXa in the physiologically relevant human prothrombinase complex. Recently a new series of specific, direct acting inhibitors of Factor Xa has been developed. And they may be more effective against Factor Xa in that they inhibit both free Factor Xa and Factor Xa in the prothrombinase complex.[7]
References:
1.K. R. Guertin et al. Optimization of the-Aminoester Class of Factor Xa Inhibitors.Part 2: Identification of FXV673 as a Potent and Selective Inhibitor with Excellent In Vivo Anticoagulant Activity. Bioorg. Med. Chem. Lett. 12 (2002) 1671–1674.
2.Meyer-Michel Samama. et al. Monitoring plasma levels of factor Xa inhibitors: how, why and when? Expert Rev. Hematol.2013, 6(2), 155-164.
3.Rebello SS1. et al. Antithrombotic efficacy of a novel factor Xa inhibitor, FXV673, in a canine model of coronary artery thrombolysis. Br J Pharmacol. 2001 Aug;133(7):1190-8.
4.Valeria Chu. et al. Pharmacological Characterization of a Novel Factor Xa Inhibitor, FXV673. Thrombosis Research 103 (2001) 309–324.
5.Katsung, B., S. Masters and A. Trevor. Basic and Clinical Pharmacology 11th Edition. United States of America: McGraw-Hill, 2009.
6.Rebello SS1. et al. Role of short-term inhibition of factor Xa by FXV673 in arterial passivation: a study in a chronic model of thrombosis in conscious dogs. J Cardiovasc Pharmacol. 2001,38(2):288-97.
7.Turpie AG. "Oral, Direct Factor Xa Inhibitors in Development for the Prevention and Treatment of Thromboembolic Diseases". Arterioscler Thromb Vasc Biol,2007,27 (6): 1238-47.
- Cardamonin
Catalog No.:BCN1184
CAS No.:19309-14-9
- CTS-1027
Catalog No.:BCC1502
CAS No.:193022-04-7
- ZM323881
Catalog No.:BCC2073
CAS No.:193001-14-8
- ZM 323881 HCl
Catalog No.:BCC5098
CAS No.:193000-39-4
- Ximelagatran
Catalog No.:BCC6382
CAS No.:192939-46-1
- LY310762
Catalog No.:BCC5052
CAS No.:192927-92-7
- 2-Amino-2'-nitrodiphenyl sulfide
Catalog No.:BCC8522
CAS No.:19284-81-2
- ES 936
Catalog No.:BCC6102
CAS No.:192820-78-3
- Cyclomulberrin
Catalog No.:BCN3374
CAS No.:19275-51-5
- Cudraflavone B
Catalog No.:BCN8067
CAS No.:19275-49-1
- 3-Isomangostin
Catalog No.:BCN1214
CAS No.:19275-46-8
- Lopinavir
Catalog No.:BCC3621
CAS No.:192725-17-0
- cis-Moschamine
Catalog No.:BCN3901
CAS No.:193224-24-7
- 1-O-Acetyl-6alpha-O-(2-methylbutyryl)britannilactone
Catalog No.:BCN7747
CAS No.:1932687-71-2
- Lonafarnib
Catalog No.:BCC2331
CAS No.:193275-84-2
- 5-Aminoindazole
Catalog No.:BCC8734
CAS No.:19335-11-6
- Co 101244 hydrochloride
Catalog No.:BCC7369
CAS No.:193356-17-1
- Tartrazine
Catalog No.:BCN2217
CAS No.:1934-21-0
- Zeylenone
Catalog No.:BCC8268
CAS No.:193410-84-3
- Fmoc-Glu(Edans)-OH
Catalog No.:BCC3491
CAS No.:193475-66-0
- Mulberroside F
Catalog No.:BCN2908
CAS No.:193483-95-3
- IPAG
Catalog No.:BCC5662
CAS No.:193527-91-2
- SB 239063
Catalog No.:BCC1923
CAS No.:193551-21-2
- Calcifediol
Catalog No.:BCC4949
CAS No.:19356-17-3
Design and rationale of the treatment of acute coronary syndromes with otamixaban trial: a double-blind triple-dummy 2-stage randomized trial comparing otamixaban to unfractionated heparin and eptifibatide in non-ST-segment elevation acute coronary syndromes with a planned early invasive strategy.[Pubmed:23194481]
Am Heart J. 2012 Dec;164(6):817-24.e13.
BACKGROUND: Otamixaban is a synthetic intravenous direct factor Xa inhibitor, with rapid onset/offset, linear kinetics, and no significant renal elimination. A phase II trial in acute coronary syndromes (ACS) showed a marked reduction in the combined end point of death or myocardial infarction (MI) and similar bleeding rates with Otamixaban at midrange doses, compared with unfractionated heparin (UFH) and eptifibatide. DESIGN: The TAO trial is a phase III, randomized, double-blind, triple-dummy controlled trial testing the efficacy of Otamixaban over UFH plus eptifibatide in patients with non-ST-segment elevation ACS to be treated with dual oral antiplatelet therapy and an invasive strategy. Approximately 13,220 patients in 55 countries will be randomized (1:1:1 ratio) to receive UFH plus downstream eptifibatide (started pre-percutaneous coronary intervention and continued per label) or Otamixaban (0.08 mg/kg intravenous bolus at randomization then 0.100 or 0.140 mg/kg per hour intravenous infusion). An interim analysis was performed after >/=1,969 patients per arm completed 7 days of follow-up and the Data Monitoring Committee selected 1 Otamixaban dose (blinded to investigators) to be carried forward using a prespecified algorithm. The primary efficacy outcome is the composite of all-cause mortality or new MI through day 7. The primary safety outcome is thrombolysis in MI major or minor bleeding through day 7. Secondary outcomes include all-cause mortality, recurrent ischemia/infarction resulting in prolonged/recurrent hospitalization, periprocedural angiographic complications, and pharmacokinetic data in 6,000 patients. CONCLUSIONS: The TAO trial will assess the clinical efficacy and safety of Otamixaban in non-ST-segment elevation ACS with planned invasive strategy.
Anticoagulation with otamixaban and ischemic events in non-ST-segment elevation acute coronary syndromes: the TAO randomized clinical trial.[Pubmed:23995608]
JAMA. 2013 Sep 18;310(11):1145-55.
IMPORTANCE: The optimal anticoagulant for patients with non-ST-segment elevation acute coronary syndromes (NSTE-ACS) managed with an invasive strategy remains controversial. OBJECTIVE: To compare the clinical efficacy and safety of Otamixaban, a novel intravenous direct factor Xa inhibitor, with that of unfractionated heparin plus downstream eptifibatide in patients with NSTE-ACS undergoing a planned early invasive strategy. DESIGN, SETTING, AND PARTICIPANTS: Randomized, double-blind, active-controlled superiority trial that enrolled 13,229 patients with NSTE-ACS and a planned early invasive strategy, at 568 active sites in 55 countries and conducted between April 2010 and February 2013. A planned interim analysis was conducted for Otamixaban dose selection. INTERVENTIONS: Eligible participants were randomized to Otamixaban (bolus and infusion, at 1 of 2 doses) or unfractionated heparin plus, at the time of percutaneous coronary intervention, eptifibatide. The Otamixaban dose selected at interim analysis was an intravenous bolus of 0.080 mg/kg followed by an infusion of 0.140 mg/kg per hour. MAIN OUTCOMES AND MEASURES: The primary efficacy outcome was the composite of all-cause death or new myocardial infarction through day 7. RESULTS: Rates of the primary efficacy outcome were 5.5% (279 of 5105 patients) randomized to receive Otamixaban and 5.7% (310 of 5466 patients) randomized to receive unfractionated heparin plus eptifibatide (adjusted relative risk, 0.99 [95% CI, 0.85-1.16]; P = .93). There were no differences for the secondary end points, including procedural thrombotic complications. The primary safety outcome of Thrombosis in Myocardial Infarction major or minor bleeding through day 7 was increased by Otamixaban (3.1% vs 1.5%; relative risk, 2.13 [95% CI, 1.63-2.78]; P < .001). Results were consistent across prespecified subgroups. CONCLUSIONS AND RELEVANCE: Otamixaban did not reduce the rate of ischemic events relative to unfractionated heparin plus eptifibatide but did increase bleeding. These findings do not support the use of Otamixaban for patients with NSTE-ACS undergoing planned early percutaneous coronary intervention. TRIAL REGISTRATION: clinicaltrials.gov Identifier: NCT01076764.


