C527Inhibitor of USP1/USF1 complex CAS# 192718-06-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 192718-06-2 | SDF | Download SDF |
| PubChem ID | 2307331 | Appearance | Powder |
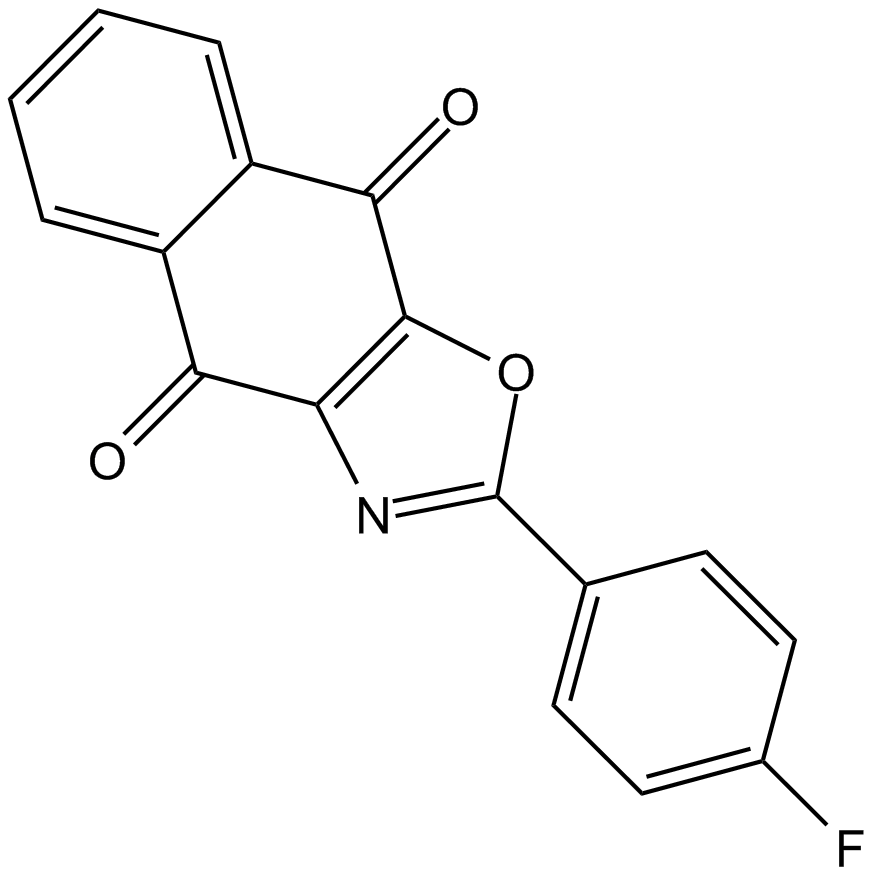
| Formula | C17H8FNO3 | M.Wt | 293.25 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : < 1 mg/mL (insoluble or slightly soluble) | ||
| Chemical Name | 2-(4-fluorophenyl)benzo[f][1,3]benzoxazole-4,9-dione | ||
| SMILES | C1=CC=C2C(=C1)C(=O)C3=C(C2=O)OC(=N3)C4=CC=C(C=C4)F | ||
| Standard InChIKey | ULJDFEYQOPCCPM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H8FNO3/c18-10-7-5-9(6-8-10)17-19-13-14(20)11-3-1-2-4-12(11)15(21)16(13)22-17/h1-8H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

C527 Dilution Calculator

C527 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
C527, also named heterocyclic tricyclic 1,4-dihydro-1,4-dioxo-1H-naphthalene, is an inhibitor of USP1 (deubiquitinating enzyme 1) / USF1 (USP1-associated factor 1) complex with IC50 value of 0.88 ± 0.03 μM in vivo. C527 is a pan-deubiquitinating enzyme inhibitor in vitro, with a high nanomolar IC50 for the USP1/UAF1 complex.
C527 inhibited the DUB activity of the USP12/USP46 complex and other DUB enzymes in vitro. However, the IC50 of C527 for these DUB enzymes was higher in comparison to USP1/UAF1 complex. C527 had considerably less inhibitory effect on UCH-L1 and UCH-L3, a different subclass of deubiquitinating enzymes, referred to as the ubiquitin C- terminal hydrolases, even though they are also cysteine proteases.
In several leukemic cell lines, C527 promote ID1 degradation, and cause cytotoxicity. In mouse osteosarcoma cells, C527 promotes the degradation of ID1 and the concurrent upregulation of p21. in human U20S osteosarcoma cells, C527 promoted the dose-dependent degradation of ID1. In Hela cells, C527 treatments caused an increase in the levels of Ub-FANCD2 and Ub-FANCI, and inhibited Camptothecin induced the Rad51 foci. Pre-treatment of hela cells with USP1 inhibitor caused an enhancement in the cytoxicity of Mitomycin C and Camptothecin .
Reference:
1.Mistry H, Hsieh G, Buhrlage SJ et al. Small-molecule inhibitors of USP1 target ID1 degradation in leukemic cells. Mol Cancer Ther. 2013 Dec;12(12):2651-62. doi: 10.1158/1535-7163.MCT-13-0103-T. Epub 2013 Oct 15.
- PD 161570
Catalog No.:BCC7765
CAS No.:192705-80-9
- H-DL-HoSer-OH
Catalog No.:BCC3242
CAS No.:1927-25-9
- 9-Benzoylcarbazole
Catalog No.:BCC8799
CAS No.:19264-68-7
- PPADS tetrasodium salt
Catalog No.:BCC6725
CAS No.:192575-19-2
- 11α-Hydroxycanrenone
Catalog No.:BCC8433
CAS No.:192569-17-8
- 9alpha,11,12-Trihydroxydrim-7-en-6-one
Catalog No.:BCN7388
CAS No.:192566-65-7
- Ergosta-4,6,8(14),22-tetraen-3-one
Catalog No.:BCN1183
CAS No.:19254-69-4
- Lomeguatrib
Catalog No.:BCC1133
CAS No.:192441-08-0
- Neuropeptide SF (human)
Catalog No.:BCC5829
CAS No.:192387-39-6
- Neuropeptide AF (human)
Catalog No.:BCC5854
CAS No.:192387-38-5
- Prazosin HCl
Catalog No.:BCC2505
CAS No.:19237-84-4
- CGP 71683 hydrochloride
Catalog No.:BCC7283
CAS No.:192322-50-2
- Lopinavir
Catalog No.:BCC3621
CAS No.:192725-17-0
- 3-Isomangostin
Catalog No.:BCN1214
CAS No.:19275-46-8
- Cudraflavone B
Catalog No.:BCN8067
CAS No.:19275-49-1
- Cyclomulberrin
Catalog No.:BCN3374
CAS No.:19275-51-5
- ES 936
Catalog No.:BCC6102
CAS No.:192820-78-3
- 2-Amino-2'-nitrodiphenyl sulfide
Catalog No.:BCC8522
CAS No.:19284-81-2
- LY310762
Catalog No.:BCC5052
CAS No.:192927-92-7
- Ximelagatran
Catalog No.:BCC6382
CAS No.:192939-46-1
- ZM 323881 HCl
Catalog No.:BCC5098
CAS No.:193000-39-4
- ZM323881
Catalog No.:BCC2073
CAS No.:193001-14-8
- CTS-1027
Catalog No.:BCC1502
CAS No.:193022-04-7
- Cardamonin
Catalog No.:BCN1184
CAS No.:19309-14-9
Laparoscopic extravesical ureteral reimplantation: technique.[Pubmed:18725984]
Adv Urol. 2008:567980.
Laparoscopic extravesical ureteral reimplantation in children is currently a technically demanding procedure with sparse literature to aid in mastering the learning curve. We present our most recent technique and lessons learned after 20 cases in children 4-15 years of age. The literature is also reviewed to encapsulate the current state-of-the-art.
The Role of the Complex USP1/WDR48 in Differentiation and Proliferation Processes in Cancer Stem Cells.[Pubmed:28302046]
Curr Stem Cell Res Ther. 2017;12(5):416-422.
BACKGROUND: Recently, some studies identified the Basic-Helix-Loop-Helix (bHLH) transcription factor as a significant regulator for the evolution of neoplasms. The binding between bHLH proteins and DNA is restricted by heterodimerization with Inhibitors of DNA binding (ID). IDs prevent cellular differentiation, promote growth and sustain tumor development. The wide presence of stem cells in cancers suggests that genes ID are essential to cancer stem cells (CSC) progress. The enzyme Ubiquitin-specific protease 1 (USP1) is reported to deubiquitinate and stabilize IDs. Considering the action of the proteins ID, USP1 contributes to prevent differentiation mediated by bHLH and, consequently, keep CSC original characteristics. USP1 has its activity potentiated when bound to protein WD repeat-containing protein (WDR48). OBJECTIVE: To identify the influence of the complex USP1/WDR48 during the CSC tumorigenesis process, and whether this complex is a possible therapeutic target. METHODS: A literature search regarding the role of the complex USP1/WDR48 in inhibiting differentiation and increasing proliferation of CSC was performed, and possible selective molecule inhibitors of these deubiquitinase proteins were investigated. RESULTS: There is evidence that USP1/WDR48 complex promotes stem cell conservation and regulation of DNA damage repair. For this reason, inhibitors as Pimozide, GW7647, C527, SJB2-043, ML323 have been studied to inhibit USPs in cases of treatment intervention. CONCLUSION: It is consolidated in the literature the role of USP1/WDR48 during tumorigenesis. However, these studies are not enough to completely clarify the process; but certainly, the researchers are converging towards a promising direction to provide a new treatment option for cancer.
Kinase activation of ClC-3 accelerates cytoplasmic condensation during mitotic cell rounding.[Pubmed:22049206]
Am J Physiol Cell Physiol. 2012 Feb 1;302(3):C527-38.
"Mitotic cell rounding" describes the rounding of mammalian cells before dividing into two daughter cells. This shape change requires coordinated cytoskeletal contraction and changes in osmotic pressure. While considerable research has been devoted to understanding mechanisms underlying cytoskeletal contraction, little is known about how osmotic gradients are involved in cell division. Here we describe cytoplasmic condensation preceding cell division, termed "premitotic condensation" (PMC), which involves cells extruding osmotically active Cl(-) via ClC-3, a voltage-gated channel/transporter. This leads to a decrease in cytoplasmic volume during mitotic cell rounding and cell division. Using a combination of time-lapse microscopy and biophysical measurements, we demonstrate that PMC involves the activation of ClC-3 by Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) in human glioma cells. Knockdown of endogenous ClC-3 protein expression eliminated CaMKII-dependent Cl(-) currents in dividing cells and impeded PMC. Thus, kinase-dependent changes in Cl(-) conductance contribute to an outward osmotic pressure in dividing cells, which facilitates cytoplasmic condensation preceding cell division.
Assessment of roles for the Rho-specific guanine nucleotide dissociation inhibitor Ly-GDI in platelet function: a spatial systems approach.[Pubmed:28148498]
Am J Physiol Cell Physiol. 2017 Apr 1;312(4):C527-C536.
On activation at sites of vascular injury, platelets undergo morphological alterations essential to hemostasis via cytoskeletal reorganizations driven by the Rho GTPases Rac1, Cdc42, and RhoA. Here we investigate roles for Rho-specific guanine nucleotide dissociation inhibitor proteins (RhoGDIs) in platelet function. We find that platelets express two RhoGDI family members, RhoGDI and Ly-GDI. Whereas RhoGDI localizes throughout platelets in a granule-like manner, Ly-GDI shows an asymmetric, polarized localization that largely overlaps with Rac1 and Cdc42 as well as microtubules and protein kinase C (PKC) in platelets adherent to fibrinogen. Antibody interference and platelet spreading experiments suggest a specific role for Ly-GDI in platelet function. Intracellular signaling studies based on interactome and pathways analyses also support a regulatory role for Ly-GDI, which is phosphorylated at PKC substrate motifs in a PKC-dependent manner in response to the platelet collagen receptor glycoprotein (GP) VI-specific agonist collagen-related peptide. Additionally, PKC inhibition diffuses the polarized organization of Ly-GDI in spread platelets relative to its colocalization with Rac1 and Cdc42. Together, our results suggest a role for Ly-GDI in the localized regulation of Rho GTPases in platelets and hypothesize a link between the PKC and Rho GTPase signaling systems in platelet function.


