LomustineAntineoplastic drug CAS# 13010-47-4 |
- Altretamine hydrochloride
Catalog No.:BCC4114
CAS No.:2975-00-0
- Palifosfamide
Catalog No.:BCC1833
CAS No.:31645-39-3
- Busulfan
Catalog No.:BCC3742
CAS No.:55-98-1
- Altretamine
Catalog No.:BCC1216
CAS No.:645-05-6
- Temozolomide
Catalog No.:BCC4386
CAS No.:85622-93-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 13010-47-4 | SDF | Download SDF |
| PubChem ID | 3950 | Appearance | Powder |
| Formula | C9H16ClN3O2 | M.Wt | 233.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (427.90 mM) *"≥" means soluble, but saturation unknown. | ||
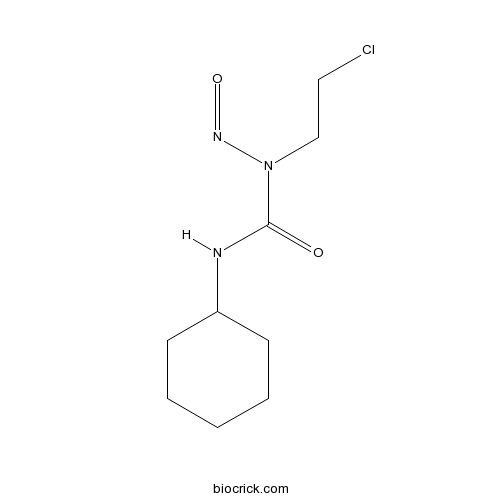
| Chemical Name | 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea | ||
| SMILES | C1CCC(CC1)NC(=O)N(CCCl)N=O | ||
| Standard InChIKey | GQYIWUVLTXOXAJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H16ClN3O2/c10-6-7-13(12-15)9(14)11-8-4-2-1-3-5-8/h8H,1-7H2,(H,11,14) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Lomustine is a DNA alkylating agent, with antitumor activity.In Vitro:Lomustine is a DNA alkylating agent. Lomustine (CCNU, 0-250 μM) is cytotoxic to the U87-MG cells expressing tumor-derived mutant IDH1, and has little effect on the expression of wild-type IDH1. The combination of Lomustine and procarbazine or vincristine has no additive effect on the killing of cells expressing mutant or wild-type IDH1. Moreover, overexpression of either ALKBH2 or ALKBH3 partially reduces the death HT1080 cells exposed to Lomustine[1]. Lomustine suppresses U87-MG growth with an ED50 of 68.1 μM. Lomustine (30, 40 μM) in combination with docosahexaenoic acid (DHA) darmatically inhibits 2 additional human-derived glioblastoma cell lines, and induces U87-MG apoptosis and necrosis. Lomustine (30 μM) causes G2/M arrest[2]. Lomustine reduces the viability of F98 rat orthotopic glioma cells and Tu-2449 mouse glioma cell line, with IC50s of 20.8 µM and 18.6 µM, respectively[3].In Vivo:Lomustine (30 mg/kg) in combination with Toca 511 + 5-FC prolongs survival in rats bearing F98 tumor cells. Lomustine (30 mg/kg) combined with Toca-511 + 5-FC also exhibits antitumor activity in the B6C3F1 mice bearing Tu-2449 glioma cells[3]. References: | |||||

Lomustine Dilution Calculator

Lomustine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.279 mL | 21.395 mL | 42.7899 mL | 85.5798 mL | 106.9748 mL |
| 5 mM | 0.8558 mL | 4.279 mL | 8.558 mL | 17.116 mL | 21.395 mL |
| 10 mM | 0.4279 mL | 2.1395 mL | 4.279 mL | 8.558 mL | 10.6975 mL |
| 50 mM | 0.0856 mL | 0.4279 mL | 0.8558 mL | 1.7116 mL | 2.1395 mL |
| 100 mM | 0.0428 mL | 0.2139 mL | 0.4279 mL | 0.8558 mL | 1.0697 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Lomustine is an antineoplastic drug used in chemotherapy [1]
Lomustine has been revealed to inhibit the growth of tumour cell lines with IC50 values of 25μM, 8.8μM and 13μM for breast ZR-75-1, astrocytoma U87MG and colorectal LS174T cell lines [2]. Besides, Lomustine has been found to be particularly effective in the treatment of certain neoplasms of the central nervous system, because of the high lipid solubility and permeability through the blood brain barrier. In addition, Lomustine has shown the effect function in treatment of meningeal leukemia in the mouse and in children who have acute leukemia with central nervous system involvement [1].
References:
[1] Cheng CJ, Fujimura S, Grunberger D, Weinstein IB. nteraction of 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (NSC 79037) with nucleic acids and proteins in vivo and in vitro. Cancer Res. 1972 Jan;32(1):22-7.
[2] Baer JC1, Freeman AA, Newlands ES, Watson AJ, Rafferty JA, Margison GP. Depletion of O6-alkylguanine-DNA alkyltransferase correlates with potentiation of temozolomide and CCNU toxicity in human tumour cells.Br J Cancer. 1993 Jun; 67(6):1299-302.
- Mafenide Acetate
Catalog No.:BCC5236
CAS No.:13009-99-9
- Dehydrocorydaline nitrate
Catalog No.:BCN2745
CAS No.:13005-09-9
- GSK1324726A
Catalog No.:BCC4038
CAS No.:1300031-52-0
- I-BET151 (GSK1210151A)
Catalog No.:BCC4476
CAS No.:1300031-49-5
- Quinine
Catalog No.:BCN2341
CAS No.:130-95-0
- Quinine HCl
Catalog No.:BCN2262
CAS No.:130-89-2
- Protopine
Catalog No.:BCN6165
CAS No.:130-86-9
- Thioridazine HCl
Catalog No.:BCC3869
CAS No.:130-61-0
- 1,4-Naphthoquinone
Catalog No.:BCN8420
CAS No.:130-15-4
- Senecionine
Catalog No.:BCN2129
CAS No.:130-01-8
- Iloperidone hydrochloride
Catalog No.:BCC4212
CAS No.:1299470-39-5
- Dapoxetine HCl
Catalog No.:BCC5064
CAS No.:129938-20-1
- (+)-Igmesine hydrochloride
Catalog No.:BCC5902
CAS No.:130152-35-1
- Ergosterol glucoside
Catalog No.:BCN7327
CAS No.:130155-33-8
- Torilolone
Catalog No.:BCN6659
CAS No.:13018-09-2
- Torilin
Catalog No.:BCN6611
CAS No.:13018-10-5
- Cirsimarin
Catalog No.:BCN6821
CAS No.:13020-19-4
- N-(4-Hydroxyphenylacetyl)spermine
Catalog No.:BCC6594
CAS No.:130210-32-1
- Acerogenin G
Catalog No.:BCN7328
CAS No.:130233-83-9
- 3'-Demethoxypiplartine
Catalog No.:BCN4021
CAS No.:130263-10-4
- PD 135158
Catalog No.:BCC7431
CAS No.:130285-87-9
- Rubiarbonol B
Catalog No.:BCN6159
CAS No.:130288-60-7
- Fmoc-Asp(OcHex)-OH
Catalog No.:BCC3468
CAS No.:130304-80-2
- HOE 140
Catalog No.:BCC5964
CAS No.:130308-48-4
Procarbazine, Lomustine, and Vincristine (PCV) Regimen for Central Nervous System Tumors.[Pubmed:28321136]
Hosp Pharm. 2017 Feb;52(2):98-104.
The complexity of cancer chemotherapy requires pharmacists be familiar with the complicated regimens and highly toxic agents used. This column reviews various issues related to preparation, dispensing, and administration of antineoplastic therapy, and the agents, both commercially available and investigational, used to treat malignant diseases. Questions or suggestions for topics should be addressed to Dominic A. Solimando, Jr, President, Oncology Pharmacy Services, Inc., 4201 Wilson Blvd #110-545, Arlington, VA 22203, e-mail: OncRxSvc@comcast.net; or J. Aubrey Waddell, Professor, University of Tennessee College of Pharmacy; Oncology Pharmacist, Pharmacy Department, Blount Memorial Hospital, 907 E. Lamar Alexander Parkway, Maryville, TN 37804, e-mail: waddfour@charter.net. The information presented in this review is based on published data and clinical expertise and includes information not included in the product labeling. Incorporation of such published data provides a more robust assessment of the drugs and assists pharmacists in evaluation of orders for off-label use of these agents.
Analysis of lomustine drug content in FDA-approved and compounded lomustine capsules.[Pubmed:28117638]
J Am Vet Med Assoc. 2017 Feb 1;250(3):322-326.
OBJECTIVE To determine the Lomustine content (potency) in compounded and FDA-approved Lomustine capsules. DESIGN Evaluation study. SAMPLE 2 formulations of Lomustine capsules (low dose [7 to 11 mg] and high dose [40 to 48 mg]; 5 capsules/dose/source) from 3 compounders and from 1 manufacturer of FDA-approved capsules. PROCEDURES Lomustine content was measured by use of a validated high-pressure liquid chromatography method. An a priori acceptable range of 90% to 110% of the stated Lomustine content was selected on the basis of US Pharmacopeia guidelines. RESULTS The measured amount of Lomustine in all compounded capsules was less than the stated content (range, 59% to 95%) and was frequently outside the acceptable range (failure rate, 2/5 to 5/5). Coefficients of variation for Lomustine content ranged from 4.1% to 16.7% for compounded low-dose capsules and from 1.1% to 10.8% for compounded high-dose capsules. The measured amount of Lomustine in all FDA-approved capsules was slightly above the stated content (range, 104% to 110%) and consistently within the acceptable range. Coefficients of variation for Lomustine content were 0.5% for low-dose and 2.3% for high-dose FDA-approved capsules. CONCLUSIONS AND CLINICAL RELEVANCE Compounded Lomustine frequently did not contain the stated content of active drug and had a wider range of Lomustine content variability than did the FDA-approved product. The sample size was small, and larger studies are needed to confirm these findings; however, we recommend that compounded veterinary formulations of Lomustine not be used when appropriate doses can be achieved with FDA-approved capsules or combinations of FDA-approved capsules.
Inhibition of histone deacetylases sensitizes glioblastoma cells to lomustine.[Pubmed:27766591]
Cell Oncol (Dordr). 2017 Feb;40(1):21-32.
PURPOSE: Glioblastoma (GBM) ranks among the deadliest solid cancers worldwide and its prognosis has remained dismal, despite the use of aggressive chemo-irradiation treatment regimens. Limited drug delivery into the brain parenchyma and frequent resistance to currently available therapies are problems that call for a prompt development of novel therapeutic strategies. While only displaying modest efficacies as mono-therapy in pre-clinical settings, histone deacetylase inhibitors (HDACi) have shown promising sensitizing effects to a number of cytotoxic agents. Here, we sought to investigate the sensitizing effect of the HDACi trichostatin A (TSA) to the alkylating agent Lomustine (CCNU), which is used in the clinic for the treatment of GBM. METHODS: Twelve primary GBM cell cultures grown as neurospheres were used in this study, as well as one established GBM-derived cell line (U87 MG). Histone deacetylase (HDAC) expression levels were determined using quantitative real-time PCR and Western blotting. The efficacy of either CCNU alone or its combination with TSA was assessed using various assays, i.e., cell viability assays (MTT), cell cycle assays (flow cytometry, FACS), double-strand DNA break (DSB) quantification assays (microscopy/immunofluorescence) and expression profiling assays of proteins involved in apoptosis and cell stress (Western blotting and protein array). RESULTS: We found that the HDAC1, 3 and 6 expression levels were significantly increased in GBM samples compared to non-neoplastic brain control samples. Additionally, we found that pre-treatment of GBM cells with TSA resulted in an enhancement of their sensitivity to CCNU, possibly via the accumulation of DSBs, decreased cell proliferation and viability rates, and an increased apoptotic rate. CONCLUSION: From our data we conclude that the combined administration of TSA and CCNU eradicates GBM cells with a higher efficacy than either drug alone, thereby opening a novel avenue for the treatment of GBM.
Is CCNU (lomustine) valuable for treatment of cutaneous epitheliotropic lymphoma in dogs? A critically appraised topic.[Pubmed:28222789]
BMC Vet Res. 2017 Feb 21;13(1):61.
BACKGROUND: CCNU and other treatment protocols are commonly offered to owners for the treatment of dogs diagnosed with cutaneous (epitheliotropic) T-cell lymphoma (CTCL). Chemotherapy protocols provide variable benefits; they have different side-effects, and they typically require monitoring to detect drug toxicity at a non-negligible cost to the owner. At this time, even though CCNU is most often recommended to treat dogs with CTCL, there is no clear consensus on the benefit of this drug. Knowing which chemotherapy protocol yields the highest rate of complete remission and longest survival times would help veterinarians and pet owners select treatment options based on the best evidence available. Our objective was to review the literature to compare the complete remission rates and survival times of CCNU-based protocols to those of other interventions. We critically assessed the data included in articles reporting treatment outcome in at least five dogs with CTCL. Single case reports and case series with less than five patients were not reviewed to avoid anecdotal evidence of lower quality. RESULTS: The search for, and review and analysis of, the best evidence available as of February 8, 2017, suggests that CCNU and pegylated liposomal doxorubicin appear to yield the highest rate of complete remission in approximately one-third of dogs with CTCL. Other treatment protocols did not report usable information on remission rates. Without any treatment, the mean/median survival time in dogs with CTCL varied between 3 and 5 months. With CCNU protocols, the median survival time was 6 months and the one with retinoids (isotretinoin and/or etretinate), PEG L-asparaginase or prednisolone monotherapy was 11, 9 and 4 months, respectively; all these durations were obtained from small numbers of dogs, however. CONCLUSIONS: CCNU leads to a complete remission of signs in approximately one-third of dogs with CTCL, but such remissions are of short duration. The median survival time after CCNU appears longer than that without treatment, but other drugs appear to provide a better long-term prognosis. Further studies are required to investigate the effect of CCNU, alone or in combination, on remission rates, survival times and impact on quality of life.


