BusulfanDNA alkylating agent CAS# 55-98-1 |
- Lomustine
Catalog No.:BCC4794
CAS No.:13010-47-4
- Altretamine hydrochloride
Catalog No.:BCC4114
CAS No.:2975-00-0
- Palifosfamide
Catalog No.:BCC1833
CAS No.:31645-39-3
- Altretamine
Catalog No.:BCC1216
CAS No.:645-05-6
- Temozolomide
Catalog No.:BCC4386
CAS No.:85622-93-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 55-98-1 | SDF | Download SDF |
| PubChem ID | 2478 | Appearance | Powder |
| Formula | C6H14O6S2 | M.Wt | 246.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
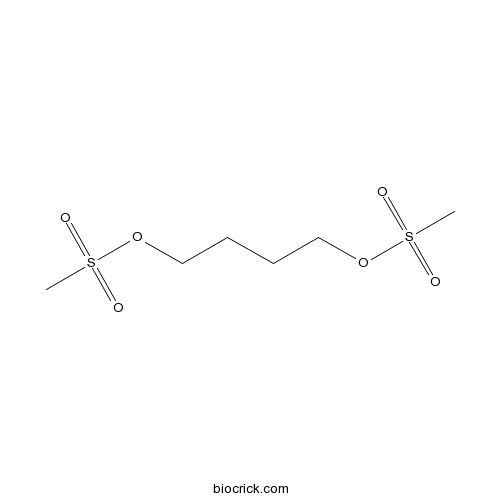
| Chemical Name | 4-methylsulfonyloxybutyl methanesulfonate | ||
| SMILES | CS(=O)(=O)OCCCCOS(=O)(=O)C | ||
| Standard InChIKey | COVZYZSDYWQREU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H14O6S2/c1-13(7,8)11-5-3-4-6-12-14(2,9)10/h3-6H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Busulfan is a potent alkylator, with chemotherapeutic activity.In Vitro:Busulfan is a potent alkylating agent, and used as a chemotherapeutic agent. Busulfan (120 μM) results in premature senescence in WI38 cells via the Erk and p38 MAPK pathway, reduces GSH and increases ROS production, but the production can be suppressed by NADPH oxidase[1].In Vivo:Busulfan (40 mg/kg, i.p) increases apoptosis and decreases the testis weight in mice. The testes of Busulfan-treated mice exhibits higher level of pRB expression, inhibits Rb phosphorylation and PCNA expression compared to the control[2]. Busulfan (2.5, 5.0 mg/kg, i.p.) causes earlier occurrence of persistent esturs in a dose dependent manner in rats. Busulfan (5.0 mg/kg) also increases the incidence of uterine adenocarcinomas and multiplicity of uterine neoplastic lesions. In addition, Busulfan decreases the serum 17β-estradiol(E2), progesterone and inhibin levels in rats, and elevates the E2/progesterone ratio only at 5.0 mg/kg[3]. References: | |||||

Busulfan Dilution Calculator

Busulfan Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0601 mL | 20.3004 mL | 40.6009 mL | 81.2018 mL | 101.5022 mL |
| 5 mM | 0.812 mL | 4.0601 mL | 8.1202 mL | 16.2404 mL | 20.3004 mL |
| 10 mM | 0.406 mL | 2.03 mL | 4.0601 mL | 8.1202 mL | 10.1502 mL |
| 50 mM | 0.0812 mL | 0.406 mL | 0.812 mL | 1.624 mL | 2.03 mL |
| 100 mM | 0.0406 mL | 0.203 mL | 0.406 mL | 0.812 mL | 1.015 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Busulfan is a DNA alkylating agent [1].
DNA alkylating agent is attached to the guanine base of DNA and stops tumor growth by crosslinking guanine nucleobases in DNA double-helix strands and causes DNA damage [1].
In normal human diploid WI38 fibroblasts, busulfan (7.5-120 μM) induced senescence in a dose-dependant way, which was associated with prolonged activation of c-Jun NH2-terminal kinase (JNK), p38 mitogen-activated protein kinase (p38) and extracellular signal-regulated kinase (Erk) [2]. The induction of senescence was initiated by the transient depletion of intracellular glutathione (GSH) and then an increase in reactive oxygen species (ROS) production, which activated the Erk and p38 MAPK pathway [1].
In the adult mouse testis, busulfan induced apoptosis and decreased testis weight. In the first week, apoptosis mainly occured to spermatogonia. In the following week, spermatogonia-specific markers Stra 8 and c-kit were reduced but Gli I remained constant, which indicated apoptosis of differentiating type A spermatogonia [3].
References:
[1]. Probin V, Wang Y, Zhou D. Busulfan-induced senescence is dependent on ROS production upstream of the MAPK pathway. Free Radic Biol Med, 2007, 42(12): 1858-1865.
[2]. Probin V, Wang Y, Bai A, et al. Busulfan selectively induces cellular senescence but not apoptosis in WI38 fibroblasts via a p53-independent but extracellular signal-regulated kinase-p38 mitogen-activated protein kinase-dependent mechanism. J Pharmacol Exp Ther, 2006, 319(2): 551-560.
[3]. Choi YJ, Ok DW, Kwon DN, et al. Murine male germ cell apoptosis induced by busulfan treatment correlates with loss of c-kit-expression in a Fas/FasL- and p53-independent manner. FEBS Lett, 2004, 575(1-3): 41-51.
- Hexamethonium Bromide
Catalog No.:BCC4561
CAS No.:55-97-0
- 1-Phenylbiguanide hydrochloride
Catalog No.:BCC6870
CAS No.:55-57-2
- Atropine sulfate
Catalog No.:BCN2716
CAS No.:55-48-1
- McN-A 343
Catalog No.:BCC7042
CAS No.:55-45-8
- Epinephrine HCl
Catalog No.:BCC4319
CAS No.:55-31-2
- Benzamide
Catalog No.:BCN5737
CAS No.:55-21-0
- HYOSCINE HYDROCHLORIDE
Catalog No.:BCN8331
CAS No.:55-16-3
- 3,3'',5-Triiodo-L-thyronine Sodium Salt
Catalog No.:BCN1419
CAS No.:55-06-1
- Shikonin acetyl
Catalog No.:BCN2452
CAS No.:54984-93-9
- Physalin D
Catalog No.:BCN7919
CAS No.:54980-22-2
- Tamoxifen Citrate
Catalog No.:BCC4382
CAS No.:54965-24-1
- Albendazole
Catalog No.:BCC3718
CAS No.:54965-21-8
- Embelin
Catalog No.:BCN2678
CAS No.:550-24-3
- Angustifoline
Catalog No.:BCN3205
CAS No.:550-43-6
- trans-Triprolidine hydrochloride
Catalog No.:BCC6742
CAS No.:550-70-9
- Afrormosine
Catalog No.:BCN3312
CAS No.:550-79-8
- Lupanine
Catalog No.:BCN5736
CAS No.:550-90-3
- Naphazoline HCl
Catalog No.:BCC4331
CAS No.:550-99-2
- NAADP tetrasodium salt
Catalog No.:BCC7808
CAS No.:5502-96-5
- (±)-Cloprostenol sodium salt
Catalog No.:BCC7315
CAS No.:55028-72-3
- Isorhamnetin-3-O-neohespeidoside
Catalog No.:BCN1234
CAS No.:55033-90-4
- Dammar-20(21)-en-3,24,25-triol
Catalog No.:BCN5734
CAS No.:55050-69-6
- 2,6,4'-Trihydroxy-4-methoxybenzophenone
Catalog No.:BCN7588
CAS No.:55051-85-9
- Protodioscin
Catalog No.:BCN6274
CAS No.:55056-80-9
High-dose chemotherapy with thiotepa, busulfan, and cyclophosphamide and autologous stem cell transplantation for patients with primary central nervous system lymphoma in first complete remission.[Pubmed:28369839]
Cancer. 2017 Aug 15;123(16):3073-3079.
BACKGROUND: High-dose chemotherapy and autologous stem cell transplantation (HDC-ASCT) is a therapeutic option for patients with primary central nervous system lymphoma (PCNSL). To the authors' knowledge, data are limited regarding its use among patients in first complete remission (CR1) with the CNS-directed conditioning regimen of thiotepa, Busulfan, and cyclophosphamide (TBC). METHODS: A retrospective analysis of patients with PCNSL in CR1 who underwent transplantation using a TBC-based conditioning regimen at 2 academic institutions was performed. RESULTS: Forty-six consecutive patients who underwent HDC-ASCT while in CR1 were identified. The most common induction regimen was high-dose methotrexate plus temozolomide and rituximab (59%). No patients received whole-brain radiotherapy. A total of 40 patients (87%) received cytarabine before undergoing ASCT as either induction intensification, early consolidation therapy, or mobilization. The median time from diagnosis to transplantation was 6 months (range, 4-15 months). The median age of the patients at the time of transplantation was 59 years (range, 27-69 years). With a median follow-up of 2.7 years after ASCT (range, 6 months-7.5 years), the Kaplan-Meier estimates of 2-year overall survival and progression-free survival were 95% (95% confidence interval [95% CI], 80%-99%) and 92% (95% CI, 77%-97%), respectively. The most common toxicities were severe mucositis (35%) and bacterial infections occurring within 100 days of transplantation (35%). The estimated 2-year nonrecurrence mortality rate was 2.9% (95% CI, 0.2%-13.4%). CONCLUSIONS: HDC-ASCT with a CNS-directed conditioning regimen such as TBC should be considered for patients with PCNSL who are in CR1 because this approach is associated with encouraging disease control and survival in this select patient population. Cancer 2017;123:3073-79. (c) 2017 American Cancer Society.
A Significant Influence of Metronidazole on Busulfan Pharmacokinetics: A Case Report of Therapeutic Drug Monitoring.[Pubmed:28328762]
Ther Drug Monit. 2017 Jun;39(3):208-210.
Busulfan is a cytotoxic agent used in preconditioning for hematopoietic stem cell transplantation. Therapeutic drug monitoring of Busulfan is necessary owing to its narrow therapeutic range. Patients undergoing preconditioning are susceptible to infection and might require coadministration of antibiotics. We present a case study of a 3-year-old girl with precursor T-cell acute lymphoblastic leukemia who received intravenous Busulfan before hematopoietic stem cell transplantation. Metronidazole was coadministered before the third dose of Busulfan because of Clostridium difficile infection. The daily pharmacokinetic analysis revealed that the clearance reduced to 57% of that before the coadministration. Although the underlying mechanism is unclear, a significant pharmacokinetic interaction was observed between Busulfan and metronidazole, underscoring the importance of therapeutic drug monitoring.
Haploidentical peripheral blood stem cell transplantation without irradiation or busulfan after reduced-intensity conditioning for KMT2A(MLL)-rearranged infant B-cell precursor acute lymphoblastic leukemia: Report of two cases.[Pubmed:28332262]
Pediatr Transplant. 2017 Jun;21(4).
We present two infants with KMT2A(MLL)-gene-R-associated BCP-ALL, who received HLA haploidentical PBSCT after RIC. The patients developed ALL at age 6 months and 3 months, respectively. Case 1 underwent PBSCT at the second CR with detectable KMT2A-AFF1(MLL-AF4) fusion gene transcript at 11 months of age, and Case 2 at the first CR without KMT2A-MLLT1(MLL-ENL) fusion gene transcript at 8 months of age. Both patients received G-CSF-mobilized unmanipulated peripheral blood mononuclear cells from their HLA haploidentical mothers after administration of FLU, MEL, and ATG. Tacrolimus, methotrexate, and mPSL were administered as prophylaxis against GVHD. Engraftment was rapidly obtained with complete chimerism in both patients. Acute adverse events included acute GVHD in Case 1 and bacterial sepsis in Case 2. At last clinical check at age 5 years and 4 years, respectively, both patients were recurrence-free and attained normal growth and development. We conclude that PBSCT from an HLA haploidentical mother with non-TBI and non-BU regimen seems feasible and efficacious, offering favorable life quality for infants.
Pediatric patients undergoing hematopoietic stem cell transplantation can greatly benefit from a novel once-daily intravenous busulfan dosing nomogram.[Pubmed:28370238]
Am J Hematol. 2017 Jul;92(7):607-613.
Busulfan, a bifunctional alkylating agent, has been used as a conditioning regimen prior to allogeneic hematopoietic stem cell transplantation (HSCT). The aim of this study was to derive a novel once-daily intravenous (IV) Busulfan dosing nomogram for pediatric patients undergoing HSCT using a population pharmacokinetic (PK) model. A population PK analysis was performed using 2183 Busulfan concentrations in 137 pediatric patients (age: 0.6-22.2 years), who received IV Busulfan once-daily for 4 days before undergoing HSCT. Based on the final population PK model, an optimal once-daily IV Busulfan dosing nomogram was derived. The percentage of simulated patients achieving the daily target area under the concentration-time curve (AUC) by the new nomogram was compared with that by other Busulfan dosing regimens including the FDA regimen, the EMA regimen, and the empirical once-daily regimen without therapeutic drug monitoring (TDM). A one-compartment open linear PK model incorporating patient's body surface area, age, dosing day, and aspartate aminotransferase as a significant covariate adequately described the concentration-time profiles of Busulfan. An optimal dosing nomogram based on the PK model performed significantly better than the other dosing regimens, resulting in >60% of patients achieving the target AUC while the percentage of patients exceeding the toxic AUC level was kept <25% during the entire treatment period. A novel once-daily Busulfan dosing nomogram for pediatric patients undergoing HSCT is useful for clinicians, particularly in a setting where TDM service is not readily available or to optimize the dose on day 1.


