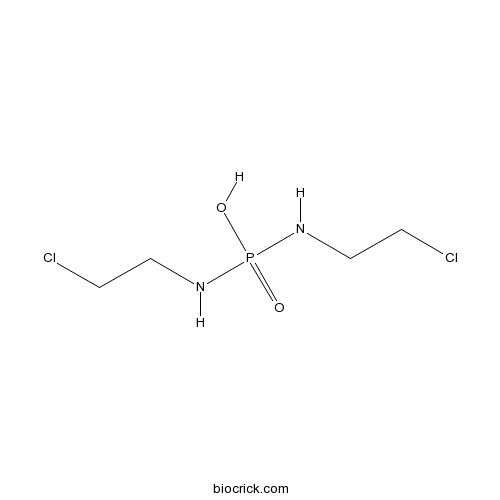
PalifosfamideActive metabolite of ifosfamide (IFOS) CAS# 31645-39-3 |
- Lomustine
Catalog No.:BCC4794
CAS No.:13010-47-4
- Altretamine hydrochloride
Catalog No.:BCC4114
CAS No.:2975-00-0
- Busulfan
Catalog No.:BCC3742
CAS No.:55-98-1
- Altretamine
Catalog No.:BCC1216
CAS No.:645-05-6
- Temozolomide
Catalog No.:BCC4386
CAS No.:85622-93-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 31645-39-3 | SDF | Download SDF |
| PubChem ID | 100427 | Appearance | Powder |
| Formula | C4H11Cl2N2O2P | M.Wt | 221.02 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Isophosphoramide mustard; IPM; ZIO-201 | ||
| Solubility | DMSO : ≥ 42 mg/mL (190.03 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | bis(2-chloroethylamino)phosphinic acid | ||
| SMILES | C(CCl)NP(=O)(NCCCl)O | ||
| Standard InChIKey | BKCJZNIZRWYHBN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C4H11Cl2N2O2P/c5-1-3-7-11(9,10)8-4-2-6/h1-4H2,(H3,7,8,9,10) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Palifosfamide is a novel DNA alkylator and the active metabolite of ifosfamide, with antitumor activity.In Vitro:Palifosfamide lysine (ZIO-201) is a stable form of palifosfamide. Palifosfamide lysine has broad activity in sarcoma lines in vitro. The IC50 ranges from 2.25 ro 6.75 μM for most cell lines except OS222 (IC50=31.5 μM)[1].In Vivo:Tumor growth inhibition is seen in both OS31 and OS33 xenografts and the RMS xenograft resulting in a significant difference in event-free survival between the control and the treated groups. Differential gene expression of ALDH3A1 but not ALDH1A1 is noted in the OS31 xenograft[1]. Stabilized palifosfamide administered to mice suppresses MX-1 tumor growth by greater than 80% with 17% complete antitumor responses. Oral bioavailability in rats is 48-73% of parenteral administration, and antitumor activity in mice is equivalent by both routes. Treatment with palifosfamide-tris combined with docetaxelor doxorubicin at optimal regimens results in complete tumor regression in 62-75% of mice[2]. References: | |||||

Palifosfamide Dilution Calculator

Palifosfamide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5245 mL | 22.6224 mL | 45.2448 mL | 90.4895 mL | 113.1119 mL |
| 5 mM | 0.9049 mL | 4.5245 mL | 9.049 mL | 18.0979 mL | 22.6224 mL |
| 10 mM | 0.4524 mL | 2.2622 mL | 4.5245 mL | 9.049 mL | 11.3112 mL |
| 50 mM | 0.0905 mL | 0.4524 mL | 0.9049 mL | 1.8098 mL | 2.2622 mL |
| 100 mM | 0.0452 mL | 0.2262 mL | 0.4524 mL | 0.9049 mL | 1.1311 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Palifosfamide is the active moiety of ifosfamide (IFA) [1].
IFA is alkylating agents which are active against a variety of pediatric sarcomas such as rhabdomyosarcoma (RMS), Ewing’s sarcoma (ES), osteosarcoma (OS) and other undifferentiated soft tissue sarcomas [1].
In human OS cell lines SaOS-2, OS229 and OS230, Palifosfamide lysine has broad activity with IC50 ranging from 2.25 to 6.75 μM. While OS222 had the IC50 of 31.5 μM [1].
In CB17 female SCID mice, palifosfamide lysine (100 mg/kg) administered intravenously for three consecutive days, the mean weight loss was less than 15% and complete recovery to baseline within 4 weeks of treatment. While, doses higher than 100 mg/kg for three consecutive days lead to either greater than 20% loss of body weight or death. In NCr-nu/nu mice bearing established orthotopic mammary MX-1 tumor xenografts, palifosfamide suppressed MX-1 tumor growth by greater than 80% with 17% complete antitumor responses [2].
References:
[1]. Hingorani P, Zhang W, Piperdi S, et al. Preclinical activity of palifosfamide lysine (ZIO-201) in pediatric sarcomas including oxazaphosphorine-resistant osteosarcoma. Cancer Chemother Pharmacol, 2009, 64(4): 733-740.
[2]. Jones B, Komarnitsky P, Miller GT, et al. Anticancer activity of stabilized palifosfamide in vivo: schedule effects, oral bioavailability, and enhanced activity with docetaxel and doxorubicin. Anticancer Drugs, 2012, 23(2): 173-184.
- 9-Anthracenylmethyl acrylate
Catalog No.:BCC8798
CAS No.:31645-34-8
- 5,7-Dihydroxy-6,8-dimethoxyflavone
Catalog No.:BCN5235
CAS No.:3162-45-6
- Artocarpesin
Catalog No.:BCN8071
CAS No.:3162-09-2
- SCH 442416
Catalog No.:BCC7372
CAS No.:316173-57-6
- Moroxydine HCl
Catalog No.:BCC4802
CAS No.:3160-91-6
- Z-Ile-OH
Catalog No.:BCC2593
CAS No.:3160-59-6
- Z-Asp(OMe)-OH
Catalog No.:BCC2790
CAS No.:3160-47-2
- Emetine dihydrochloride
Catalog No.:BCN8307
CAS No.:316-42-7
- Berberine Sulphate
Catalog No.:BCC8131
CAS No.:316-41-6
- Heraclenol
Catalog No.:BCN5234
CAS No.:31575-93-6
- 4EGI-1
Catalog No.:BCC5337
CAS No.:315706-13-9
- PTC-209
Catalog No.:BCC5111
CAS No.:315704-66-6
- 6-Aminonicotinic acid
Catalog No.:BCC8764
CAS No.:3167-49-5
- Gatifloxacin mesylate
Catalog No.:BCC4225
CAS No.:316819-28-0
- Pinusolide
Catalog No.:BCN5236
CAS No.:31685-80-0
- Levomefolic acid
Catalog No.:BCC1703
CAS No.:31690-09-2
- Aminophylline
Catalog No.:BCC2300
CAS No.:317-34-0
- Hesperetin 7-O-glucoside
Catalog No.:BCN5237
CAS No.:31712-49-9
- 5,7-Dihydroxychromone
Catalog No.:BCN4652
CAS No.:31721-94-5
- 3,5,7-Trihydroxychromone
Catalog No.:BCN7479
CAS No.:31721-95-6
- GW501516
Catalog No.:BCC2268
CAS No.:317318-70-0
- GW0742
Catalog No.:BCC2267
CAS No.:317318-84-6
- O-1602
Catalog No.:BCC7487
CAS No.:317321-41-8
- TCS 2314
Catalog No.:BCC6080
CAS No.:317353-73-4
Preclinical activity of palifosfamide lysine (ZIO-201) in pediatric sarcomas including oxazaphosphorine-resistant osteosarcoma.[Pubmed:19224214]
Cancer Chemother Pharmacol. 2009 Sep;64(4):733-40.
PURPOSE: Oxazaphosphorines, such as ifosfamide (IFA), are frequently used in the treatment of pediatric sarcomas. They are pro-drugs and undergo hepatic metabolism into the active moiety and potentially toxic by-products such as acrolein and chloracetaldehyde, which may cause hemorrhagic cystitis and encephalopathy, respectively. In addition, resistance to oxazaphosphorines can be mediated by overexpression of enzymes involved in their catabolism. Isophosphoramide mustard (IPM, Palifosfamide) is the active moiety of IFA. In the current study, the activity of Palifosfamide lysine (ZIO-201), a stable form of Palifosfamide, was evaluated in a panel of sarcoma cell lines and tumor xenografts including oxazaphosphorine-resistant xenografts. METHODS: The cytotoxic effect of Palifosfamide lysine was studied in osteosarcoma (OS), Ewing's sarcoma (ES) and rhabdomyosarcoma (RMS) cell lines using the MTT assay. In vivo, the maximum tolerated dose (MTD) of Palifosfamide lysine was determined in SCID mice based on a 3-day intravenous (IV) administration schedule. The effect on tumor growth and event-free survival was assessed at the MTD in all three sarcoma xenografts. In OS, cyclophosphamide (CPA)-resistant and -sensitive xenografts (OS31 and OS33, respectively) were evaluated for Palifosfamide lysine activity. ALDH1A1 and ALDH3A1 gene expression data for the OS xenografts were mined from the Pediatric Preclinical Testing Program gene expression data. ALDH3A1 enzyme levels were compared between the CPA-resistant and -sensitive xenografts. RESULTS: Palifosfamide lysine was cytotoxic against all the cell lines tested with the IC(50) ranging from 0.5 to 1.5 microg/ml except for OS222, which had an IC(50) of 7 microg/ml. The IV MTD of Palifosfamide lysine in mice was 100 mg/kg per day for three consecutive days. Tumor growth inhibition was seen in both OS31 and OS33 xenografts and the RMS xenograft resulting in a significant difference in event-free survival between the control and the treated groups. Differential gene expression of ALDH3A1 but not ALDH1A1 was noted in the OS31 xenograft. This was confirmed by RT-PCR and the ALDH3A1 enzyme assay. ALDH3A1 enzyme activity was measured at 100 mIU/mg of protein in OS31 xenograft but no significant activity was seen in the OS33 xenograft. CONCLUSIONS: We conclude that Palifosfamide lysine has broad activity in a panel of sarcoma cell lines. It inhibits tumor growth in OS and RMS xenografts. Furthermore, it is active against the CPA-resistant, ALDH3A1 overexpressing, OS xenograft suggesting that it might have the potential of overcoming this resistance mechanism against oxazaphosphorines and may be an active agent in resistant/relapsed sarcomas in patients.
PICASSO III: A Phase III, Placebo-Controlled Study of Doxorubicin With or Without Palifosfamide in Patients With Metastatic Soft Tissue Sarcoma.[Pubmed:27621408]
J Clin Oncol. 2016 Nov 10;34(32):3898-3905.
Purpose Palifosfamide is the active metabolite of ifosfamide and does not require prodrug activation, thereby avoiding the generation of toxic metabolites. The PICASSO III trial compared doxorubicin plus Palifosfamide with doxorubicin plus placebo in patients who had received no prior systemic therapy for metastatic soft tissue sarcoma. Patients and Methods Patients were randomly assigned 1:1 to receive doxorubicin 75 mg/m(2) intravenously day 1 plus Palifosfamide 150 mg/m(2)/d intravenously days 1 to 3 or doxorubicin plus placebo once every 21 days for up to six cycles. The primary end point was progression-free survival (PFS) by independent radiologic review. Results In all, 447 patients were randomly assigned to receive doxorubicin plus Palifosfamide (n = 226) or doxorubicin plus placebo (n = 221). Median PFS was 6.0 months for doxorubicin plus Palifosfamide and 5.2 months for doxorubicin plus placebo (hazard ratio, 0.86; 95% CI, 0.68 to 1.08; P = .19). Median overall survival was 15.9 months for doxorubicin plus Palifosfamide and 16.9 months for doxorubicin plus placebo (hazard ratio, 1.05; 95% CI, 0.79 to 1.39; P = .74). There was a higher incidence of grade 3 to 4 adverse events in the doxorubicin plus Palifosfamide arm (63.6% v 50.9%) including a higher rate of febrile neutropenia (21.4% v 12.6%). Conclusion No significant difference in PFS was observed in patients receiving doxorubicin plus Palifosfamide compared with those receiving doxorubicin plus placebo. The observed median PFS and overall survival in this large, international study can serve as a benchmark for future studies of doxorubicin in metastatic soft tissue sarcoma.
Anticancer activity of stabilized palifosfamide in vivo: schedule effects, oral bioavailability, and enhanced activity with docetaxel and doxorubicin.[Pubmed:22027537]
Anticancer Drugs. 2012 Feb;23(2):173-84.
Palifosfamide, the DNA-alkylating metabolite of ifosfamide (IFOS), has been synthesized as a stabilized tris or lysine salt and found to have preclinical and clinical antitumor activity. Stabilized Palifosfamide overcomes limitations of IFOS because of patient-to-patient variability in response resulting from variable prodrug activation, resistance and toxicities of metabolic byproducts, acrolein and chloroacetaldehyde. Palifosfamide represents an effective alternative to IFOS and other DNA-alkylating prodrugs. The antitumor activities of stabilized Palifosfamide were investigated in vivo. Dose response, route and schedule of administration, and interaction with docetaxel or doxorubicin were investigated in NCr-nu/nu mice bearing established orthotopic mammary MX-1 tumor xenografts. Oral activity was investigated in P388-1 leukemia in CD2F1 mice. Oral and intraperitoneal bioavailabilities were compared in Sprague-Dawley rats. Stabilized Palifosfamide administered by optimized regimens suppressed MX-1 tumor growth (P<0.05) by greater than 80% with 17% complete antitumor responses and up to three-fold increase in time to three tumor doublings over controls. Median survival in the P388-1 (P<0.001) model was increased by 9 days over controls. Oral bioavailability in rats was 48-73% of parenteral administration, and antitumor activity in mice was equivalent by both routes. Treatment with Palifosfamide-tris combined with docetaxel or doxorubicin at optimal regimens resulted in complete tumor regression in 62-75% of mice. These studies support investigation of stabilized Palifosfamide in human cancers by parenteral or oral administration as a single agent and in combination with other approved drugs. The potential for clinical translation of the cooperative interaction of Palifosfamide-tris with doxorubicin by intravenous administration is supported by results from a recent randomized Phase-II study in unresectable or metastatic soft-tissue sarcoma.
Palifosfamide, a bifunctional alkylator for the treatment of sarcomas.[Pubmed:20024846]
IDrugs. 2010 Jan;13(1):38-48.
Ifosfamide is a chemotherapeutic prodrug used in the treatment of several tumor entities, including bone and soft-tissue sarcoma. However, the application of high-dose ifosfamide is not feasible because of severe side effects caused by metabolites. The active metabolite isophosphoramide mustard is not suitable for administration because of chemical instability. ZIOPHARM Oncology Inc, under license from Dekk-Tec Inc, is developing Palifosfamide, a formulation of isophosphoramide mustard with tris(hydroxymethyl)aminomethane salt-stabilization (Palifosfamide-tris) and previously with lysine-stabilization (Palifosfamide-lys). Preclinical studies and phase I and I/II clinical trials demonstrated that Palifosfamide-tris had an antitumor efficiency comparable or superior to that of ifosfamide. Patients treated with Palifosfamide-tris did not display any of the neurotoxic or nephrotoxic side effects associated with ifosfamide. At the time of publication, data from phase II trials were being evaluated and phase III trials were being planned. Palifosfamide-tris is expected to be a safer and less toxic alternative to ifosfamide; however, considering other new approaches under investigation for tumors such as sarcoma, such as molecular-based treatment strategies, it is unclear what position Palifosfamide-tris might occupy on the market.


