GW501516PPARβ agonist,selective and potent CAS# 317318-70-0 |
- Rosiglitazone maleate
Catalog No.:BCC2262
CAS No.:155141-29-0
- Gemfibrozil
Catalog No.:BCC4783
CAS No.:25812-30-0
- Rosiglitazone HCl
Catalog No.:BCC2269
CAS No.:302543-62-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 317318-70-0 | SDF | Download SDF |
| PubChem ID | 9803963 | Appearance | Powder |
| Formula | C21H18F3NO3S2 | M.Wt | 453.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GW 1516; GSK-516 | ||
| Solubility | DMSO : ≥ 100 mg/mL (220.51 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
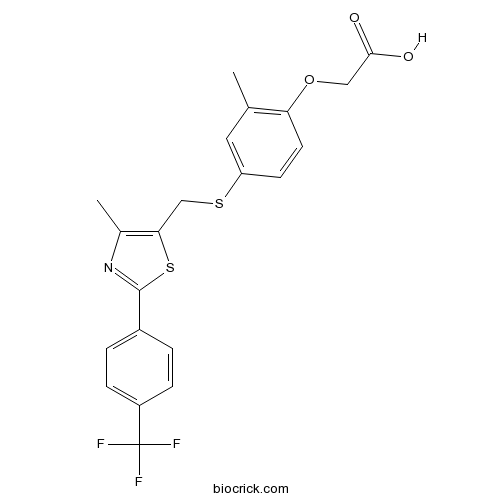
| Chemical Name | 2-[2-methyl-4-[[4-methyl-2-[4-(trifluoromethyl)phenyl]-1,3-thiazol-5-yl]methylsulfanyl]phenoxy]acetic acid | ||
| SMILES | CC1=C(C=CC(=C1)SCC2=C(N=C(S2)C3=CC=C(C=C3)C(F)(F)F)C)OCC(=O)O | ||
| Standard InChIKey | YDBLKRPLXZNVNB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H18F3NO3S2/c1-12-9-16(7-8-17(12)28-10-19(26)27)29-11-18-13(2)25-20(30-18)14-3-5-15(6-4-14)21(22,23)24/h3-9H,10-11H2,1-2H3,(H,26,27) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective PPARδ agonist (EC50 = 1.2 nM). Displays <1000-fold selectivity over other ppar subtypes. increases abc a1 transporter expression and induces apolipoprotein a1-mediated cholesterol efflux in vitro. Also increase serum HDL cholesterol and lowers small, dense LDL levels in obesity in vivo models. |

GW501516 Dilution Calculator

GW501516 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2051 mL | 11.0254 mL | 22.0507 mL | 44.1014 mL | 55.1268 mL |
| 5 mM | 0.441 mL | 2.2051 mL | 4.4101 mL | 8.8203 mL | 11.0254 mL |
| 10 mM | 0.2205 mL | 1.1025 mL | 2.2051 mL | 4.4101 mL | 5.5127 mL |
| 50 mM | 0.0441 mL | 0.2205 mL | 0.441 mL | 0.882 mL | 1.1025 mL |
| 100 mM | 0.0221 mL | 0.1103 mL | 0.2205 mL | 0.441 mL | 0.5513 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GW501516 is subtype-selective small-molecule agonist of peroxisome proliferator-activated receptor δ (PPARδ) with Ki value of 1.1nM [1].
GW501516 is discovered by combinatorial chemistry and structure-based drug design to use as an ideal chemical tool to study the function of the ubiquitously expressed PPARδ. In the cell-based transfection assay, it induces expression of a GAL4-responsive reporter gene with EC50 value of 1.2nM. GW501516 shows more than 1000-fold selective for PPARδ over other subtypes. PPARδ is expressed in many tissues that contribute to cholesterol flux, and is a RXR of partner. As an agonist of PPARδ, GW501516 is found to increase the expression of ABCA1 as well as the apoA1-specific cholesterol efflux. In primate model of human metabolic disease, GW501516 increases the level of HDLc and lowers the fasting triglycerides. GW501516 also produces fewer, yet larger, LDL and VLDL particles results in a less atherogenic lipid composition. Furthermore, GW501516 partially corrects the hyperinsulinemia in primates without adverse effects on glycemic control [1, 2].
References:
[1] Wei ZL, Kozikowski AP. A short and efficient synthesis of the pharmacological research tool GW501516 for the peroxisome proliferator-activated receptor delta. J Org Chem. 2003 Nov 14;68(23):9116-8.
[2] Oliver WR Jr, Shenk JL, Snaith MR, Russell CS, Plunket KD, Bodkin NL, Lewis MC, Winegar DA, Sznaidman ML, Lambert MH, Xu HE, Sternbach DD, Kliewer SA, Hansen BC, Willson TM. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci U S A. 2001 Apr 24;98(9):5306-11.
- 3,5,7-Trihydroxychromone
Catalog No.:BCN7479
CAS No.:31721-95-6
- 5,7-Dihydroxychromone
Catalog No.:BCN4652
CAS No.:31721-94-5
- Hesperetin 7-O-glucoside
Catalog No.:BCN5237
CAS No.:31712-49-9
- Aminophylline
Catalog No.:BCC2300
CAS No.:317-34-0
- Levomefolic acid
Catalog No.:BCC1703
CAS No.:31690-09-2
- Pinusolide
Catalog No.:BCN5236
CAS No.:31685-80-0
- Gatifloxacin mesylate
Catalog No.:BCC4225
CAS No.:316819-28-0
- 6-Aminonicotinic acid
Catalog No.:BCC8764
CAS No.:3167-49-5
- Palifosfamide
Catalog No.:BCC1833
CAS No.:31645-39-3
- 9-Anthracenylmethyl acrylate
Catalog No.:BCC8798
CAS No.:31645-34-8
- 5,7-Dihydroxy-6,8-dimethoxyflavone
Catalog No.:BCN5235
CAS No.:3162-45-6
- Artocarpesin
Catalog No.:BCN8071
CAS No.:3162-09-2
- GW0742
Catalog No.:BCC2267
CAS No.:317318-84-6
- O-1602
Catalog No.:BCC7487
CAS No.:317321-41-8
- TCS 2314
Catalog No.:BCC6080
CAS No.:317353-73-4
- 2-Methyl-4-(2-methylbenzoylamino)benzoic acid
Catalog No.:BCC8579
CAS No.:317374-08-6
- 3-Deoxyaconitine
Catalog No.:BCN2797
CAS No.:3175-95-9
- (-)-Lyoniresinol
Catalog No.:BCN3488
CAS No.:31768-94-2
- H-Ala-pNA.HCl
Catalog No.:BCC3195
CAS No.:31796-55-1
- Propranolol HCl
Catalog No.:BCC4336
CAS No.:318-98-9
- H-Phe-OEt.HCl
Catalog No.:BCC3007
CAS No.:3182-93-2
- H-Phenylalaninol
Catalog No.:BCC2719
CAS No.:3182-95-4
- H-Orn-OH. HCl
Catalog No.:BCC3000
CAS No.:3184-13-2
- Methyl orsellinate
Catalog No.:BCN5238
CAS No.:3187-58-4
Apoptotic effect of the selective PPARbeta/delta agonist GW501516 in invasive bladder cancer cells.[Pubmed:27638828]
Tumour Biol. 2016 Nov;37(11):14789-14802.
GW501516 is a selective and high-affinity synthetic agonist of peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta). This molecule promoted the inhibition of proliferation and apoptosis in few cancer cell lines, but its anticancer action has never been investigated in bladder tumor cells. Thus, this study was undertaken to determine whether GW501516 had antiproliferative and/or apoptotic effects on RT4 and T24 urothelial cancer cells and to explore the molecular mechanisms involved. Our results indicated that, in RT4 cells (derived from a low-grade papillary tumor), GW501516 did not induce cell death. On the other hand, in T24 cells (derived from an undifferentiated high-grade carcinoma), this PPARbeta/delta agonist induced cytotoxic effects including cell morphological changes, a decrease of cell viability, a G2/M cell cycle arrest, and the cell death as evidenced by the increase of the sub-G1 cell population. Furthermore, GW501516 triggered T24 cell apoptosis in a caspase-dependent manner including both extrinsic and intrinsic apoptotic pathways through Bid cleavage. In addition, the drug led to an increase of the Bax/Bcl-2 ratio, a mitochondrial dysfunction associated with the dissipation of DeltaPsim, and the release of cytochrome c from the mitochondria to the cytosol. GW501516 induced also ROS generation which was not responsible for T24 cell death since NAC did not rescue cells upon PPARbeta/delta agonist exposure. For the first time, our data highlight the capacity of GW501516 to induce apoptosis in invasive bladder cancer cells. This molecule could be relevant as a therapeutic drug for high-grade urothelial cancers.
A metabolomic study of the PPARdelta agonist GW501516 for enhancing running endurance in Kunming mice.[Pubmed:25943561]
Sci Rep. 2015 May 6;5:9884.
Exercise can increase peroxisome proliferator-activated receptor-delta (PPARdelta) expression in skeletal muscle. PPARdelta regulates muscle metabolism and reprograms muscle fibre types to enhance running endurance. This study utilized metabolomic profiling to examine the effects of GW501516, a PPARdelta agonist, on running endurance in mice. While training alone increased the exhaustive running performance, GW501516 treatment enhanced running endurance and the proportion of succinate dehydrogenase (SDH)-positive muscle fibres in both trained and untrained mice. Furthermore, increased levels of intermediate metabolites and key enzymes in fatty acid oxidation pathways were observed following training and/or treatment. Training alone increased serum inositol, glucogenic amino acids, and branch chain amino acids. However, GW501516 increased serum galactose and beta-hydroxybutyrate, independent of training. Additionally, GW501516 alone raised serum unsaturated fatty acid levels, especially polyunsaturated fatty acids, but levels increased even more when combined with training. These findings suggest that mechanisms behind enhanced running capacity are not identical for GW501516 and training. Training increases energy availability by promoting catabolism of proteins, and gluconeogenesis, whereas GW501516 enhances specific consumption of fatty acids and reducing glucose utilization.
A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport.[Pubmed:11309497]
Proc Natl Acad Sci U S A. 2001 Apr 24;98(9):5306-11.
The peroxisome proliferator-activated receptors (PPARs) are dietary lipid sensors that regulate fatty acid and carbohydrate metabolism. The hypolipidemic effects of the fibrate drugs and the antidiabetic effects of the glitazone drugs in humans are due to activation of the alpha (NR1C1) and gamma (NR1C3) subtypes, respectively. By contrast, the therapeutic potential of the delta (NR1C2) subtype is unknown, due in part to the lack of selective ligands. We have used combinatorial chemistry and structure-based drug design to develop a potent and subtype-selective PPARdelta agonist, GW501516. In macrophages, fibroblasts, and intestinal cells, GW501516 increases expression of the reverse cholesterol transporter ATP-binding cassette A1 and induces apolipoprotein A1-specific cholesterol efflux. When dosed to insulin-resistant middle-aged obese rhesus monkeys, GW501516 causes a dramatic dose-dependent rise in serum high density lipoprotein cholesterol while lowering the levels of small-dense low density lipoprotein, fasting triglycerides, and fasting insulin. Our results suggest that PPARdelta agonists may be effective drugs to increase reverse cholesterol transport and decrease cardiovascular disease associated with the metabolic syndrome X.


