PinusolideCAS# 31685-80-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 31685-80-0 | SDF | Download SDF |
| PubChem ID | 161721 | Appearance | Powder |
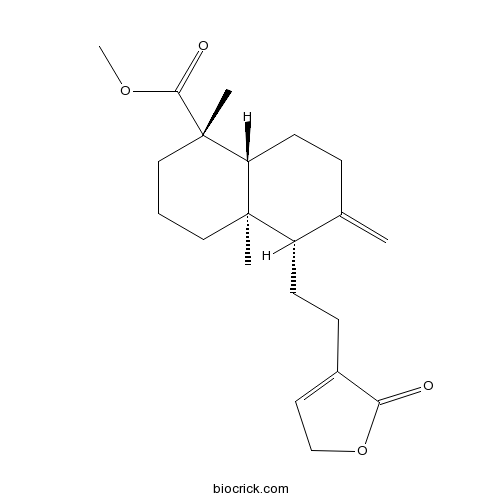
| Formula | C21H30O4 | M.Wt | 346.5 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | methyl (1S,4aR,5S,8aR)-1,4a-dimethyl-6-methylidene-5-[2-(5-oxo-2H-furan-4-yl)ethyl]-3,4,5,7,8,8a-hexahydro-2H-naphthalene-1-carboxylate | ||
| SMILES | CC12CCCC(C1CCC(=C)C2CCC3=CCOC3=O)(C)C(=O)OC | ||
| Standard InChIKey | WTKBZJAWPZXKJU-NLEAXPPASA-N | ||
| Standard InChI | InChI=1S/C21H30O4/c1-14-6-9-17-20(2,11-5-12-21(17,3)19(23)24-4)16(14)8-7-15-10-13-25-18(15)22/h10,16-17H,1,5-9,11-13H2,2-4H3/t16-,17+,20+,21-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Pinusolide is a platelet activating factor ( PAF) antagonist, it may prove of therapeutic value in the treatment of hypotension . 2. Pinusolide has antileukemic potential, it not only decreases the proliferation activity of tumor cells at relatively low concentrations but specifically induces apoptosis at 100 microM via the mitochondrial pathway in the Burkitt lymphoma cell line BJAB. 3. Pinusolide attenuates blockade of insulin signaling by enhancing IRS-1 tyrosine phosphorylation by the activating the AMPK pathway, indicates the targeting of AMPK represents a new therapeutic strategy for hyperglycemia-induced insulin resistance and type 2 diabetes. 4. Pinusolide can protect neuronal cells from staurosporine (STS) -induced apoptosis, probably by preventing the increase in [Ca2+]i and cellular oxidation caused by STS, and indicate that it could be used to treat neurodegenerative diseases. 5. Pinusolide shows potent inhibition of 5-LO dependent LTC4 generation, which requires both suppression of calcium influx and JNK phosphorylation. |
| Targets | AMPK | LOX | Calcium Channel | JNK | ERK | p38MAPK | Caspase |

Pinusolide Dilution Calculator

Pinusolide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.886 mL | 14.43 mL | 28.86 mL | 57.7201 mL | 72.1501 mL |
| 5 mM | 0.5772 mL | 2.886 mL | 5.772 mL | 11.544 mL | 14.43 mL |
| 10 mM | 0.2886 mL | 1.443 mL | 2.886 mL | 5.772 mL | 7.215 mL |
| 50 mM | 0.0577 mL | 0.2886 mL | 0.5772 mL | 1.1544 mL | 1.443 mL |
| 100 mM | 0.0289 mL | 0.1443 mL | 0.2886 mL | 0.5772 mL | 0.7215 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Gatifloxacin mesylate
Catalog No.:BCC4225
CAS No.:316819-28-0
- 6-Aminonicotinic acid
Catalog No.:BCC8764
CAS No.:3167-49-5
- Palifosfamide
Catalog No.:BCC1833
CAS No.:31645-39-3
- 9-Anthracenylmethyl acrylate
Catalog No.:BCC8798
CAS No.:31645-34-8
- 5,7-Dihydroxy-6,8-dimethoxyflavone
Catalog No.:BCN5235
CAS No.:3162-45-6
- Artocarpesin
Catalog No.:BCN8071
CAS No.:3162-09-2
- SCH 442416
Catalog No.:BCC7372
CAS No.:316173-57-6
- Moroxydine HCl
Catalog No.:BCC4802
CAS No.:3160-91-6
- Z-Ile-OH
Catalog No.:BCC2593
CAS No.:3160-59-6
- Z-Asp(OMe)-OH
Catalog No.:BCC2790
CAS No.:3160-47-2
- Emetine dihydrochloride
Catalog No.:BCN8307
CAS No.:316-42-7
- Berberine Sulphate
Catalog No.:BCC8131
CAS No.:316-41-6
- Levomefolic acid
Catalog No.:BCC1703
CAS No.:31690-09-2
- Aminophylline
Catalog No.:BCC2300
CAS No.:317-34-0
- Hesperetin 7-O-glucoside
Catalog No.:BCN5237
CAS No.:31712-49-9
- 5,7-Dihydroxychromone
Catalog No.:BCN4652
CAS No.:31721-94-5
- 3,5,7-Trihydroxychromone
Catalog No.:BCN7479
CAS No.:31721-95-6
- GW501516
Catalog No.:BCC2268
CAS No.:317318-70-0
- GW0742
Catalog No.:BCC2267
CAS No.:317318-84-6
- O-1602
Catalog No.:BCC7487
CAS No.:317321-41-8
- TCS 2314
Catalog No.:BCC6080
CAS No.:317353-73-4
- 2-Methyl-4-(2-methylbenzoylamino)benzoic acid
Catalog No.:BCC8579
CAS No.:317374-08-6
- 3-Deoxyaconitine
Catalog No.:BCN2797
CAS No.:3175-95-9
- (-)-Lyoniresinol
Catalog No.:BCN3488
CAS No.:31768-94-2
Pinusolide isolated from Biota orientalis inhibits 5-lipoxygenase dependent leukotriene C4 generation by blocking c-Jun N-terminal kinase pathway in mast cells.[Pubmed:22863941]
Biol Pharm Bull. 2012;35(8):1374-8.
Pinusolide, an herbal medicine isolated from Biota orientalis L. (B. orientalis), inhibited 5-lipoxygenase (5-LO)-dependent leukotriene C4 (LTC4) generation in immunoglobulin E (IgE)/Ag-induced bone marrow-derived mast cells (BMMCs) in a concentration-dependent manner. To clarify the action mechanism of Pinusolide on the inhibition of LTC4 generation, we examined the effect of Pinusolide on phosphorylation of cytosolic phospholipase A2 (cPLA2), as well as translocation phospho-cPLA2 and 5-LO to nucleus. Inhibition of LTC4 generation by Pinusolide was accompanied by a decrease in cPLA2 phosphorylation which occurred via a decrease in intracellular Ca2+ influx and blocking the c-Jun N-terminal kinase (JNK) pathways. However, Pinusolide had no effect on extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein (MAP) kinas phosphorylation. Taken together, the present results suggest that potent inhibition of 5-LO dependent LTC4 generation by Pinusolide requires both suppression of calcium influx and JNK phosphorylation.
Pinusolide from the leaves of Biota orientalis as potent platelet activating factor antagonist.[Pubmed:10083843]
Planta Med. 1999 Feb;65(1):39-42.
We investigated the effect of a new PAF antagonist Pinusolide, isolated from the leaves of Biota orientalis, on PAF-induced [3H]serotinin release from rabbit platelets, hypotension and vascular permeability. Pinusolide (IC50, about 5 x 10(-6) M) inhibited specifically [3H]serotinin release from rabbit platelets when stimulated with PAF (5 x 10(-8) M), but showed no effect when induced by ADP, collagen, and thrombin. It also inhibited PAF-induced hypotension in a dose-dependent manner in rats with no effect on the hypotension induced by acetylcholine, histamine and serotonin. The inhibitory effect of Pinusolide on the PAF-induced vascular permeability is less specific than the induced hypotension. These results suggest that Pinusolide may prove of therapeutic value in the treatment of hypotension and a molecular design of Pinusolide analogues may provide the possibility of a new PAF specific antagonists.
Pinusolide improves high glucose-induced insulin resistance via activation of AMP-activated protein kinase.[Pubmed:23831466]
Biochem Biophys Res Commun. 2013 Aug 2;437(3):374-9.
Adenosine monophosphate (AMP)-activated protein kinase (AMPK) plays a crucial role in the maintenance of cellular energy homeostasis, and several natural compounds that activate AMPK possibly enhance glucose uptake by muscle cells. In this study, we found that Pinusolide stimulated AMPK phosphorylation and glucose uptake and these effects were significantly reduced by siRNA LKB1 or compound C, suggesting that enhanced glucose uptake by Pinusolide is predominantly accomplished via an LKB1-mediated AMPK activation pathway. An insulin resistance state was induced by exposing cells to 30mM glucose, as indicated by reduced insulin-stimulated tyrosine phosphorylation of IRS-1 and glucose uptake. Under these conditions, the phosphorylation of AMPK and ACC were decreased. Surprisingly, disrupted insulin signaling and decreased AMPK activity by high glucose concentrations were prevented by Pinusolide. Moreover, this treatment increased insulin-stimulated glucose uptake via AMPK activation. Taken together, our findings suggest a link between high glucose and insulin resistance in muscle cells, and provide further evidence that Pinusolide attenuates blockade of insulin signaling by enhancing IRS-1 tyrosine phosphorylation by the activating the AMPK pathway. In addition, this study indicates the targeting of AMPK represents a new therapeutic strategy for hyperglycemia-induced insulin resistance and type 2 diabetes.
Gram-scale synthesis of pinusolide and evaluation of its antileukemic potential.[Pubmed:16781150]
Bioorg Med Chem Lett. 2006 Aug 15;16(16):4228-32.
Pinusolide (1), a known platelet-activating factor (PAF) receptor binding antagonist, was synthesized from lambertianic acid (2), a labdane-type diterpene readily accessible in multigram quantities from the Siberian pine tree. It was shown that 1 not only decreases the proliferation activity of tumor cells at relatively low concentrations but specifically induces apoptosis at 100 microM via the mitochondrial pathway in the Burkitt lymphoma cell line BJAB. Also, using primary lymphoblasts and leukemic cells from children with acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML), a significant DNA fragmentation in Pinusolide-treated cells could be detected in an ex vivo apoptosis assay.
Pinusolide and 15-methoxypinusolidic acid attenuate the neurotoxic effect of staurosporine in primary cultures of rat cortical cells.[Pubmed:17143305]
Br J Pharmacol. 2007 Jan;150(1):65-71.
BACKGROUND AND PURPOSE: Apoptosis is a fundamental process required for neuronal development but also occurs in most of the common neurodegenerative disorders. In an attempt to obtain an anti-apoptotic neuroprotective compound from natural products, we isolated the diterpenoids, Pinusolide and 15-MPA, from B. orientalis and investigated their neuroprotective activity against staurosporine (STS) -induced neuronal apoptosis. In addition, we determined the anti-apoptotic mechanism of these compounds in rat cortical cells. EXPERIMENTAL APPROACH: Primary cultures of rat cortical cells injured by STS were used as an in vitro assay system. Cells were pretreated with Pinusolide or 15-MPA before exposure to STS. Anti-apoptotic activities were evaluated by the measurement of cytoplasmic condensation and nuclear fragmentation. The levels of cellular peroxide, malondialdehyde (MDA) and [Ca(2+)]i, as well as the activities of superoxide dismutase (SOD) and caspase-3/7, were measured. KEY RESULTS: Pinusolide and 15-MPA, at a concentration of 5.0 iM, reduced the condensed nuclei and rise in [Ca(2+)]i that accompanies apoptosis induced by 100 nM STS. Pinusolide and 15-MPA also protected the cellular activity of SOD, an antioxidative enzyme reduced by STS insult. Furthermore, the overproduction of reactive oxygen species and lipid peroxidation induced by STS was significantly reduced in Pinusolide and 15-MPA treated cells. In addition, Pinusolide and 15-MPA inhibited STS-induced caspase-3/7 activation. CONCLUSIONS AND IMPLICATIONS: These results show that Pinusolide and 15-MPA protect neuronal cells from STS-induced apoptosis, probably by preventing the increase in [Ca(2+)]i and cellular oxidation caused by STS, and indicate that they could be used to treat neurodegenerative diseases.


