5,7-DihydroxychromoneCAS# 31721-94-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 31721-94-5 | SDF | Download SDF |
| PubChem ID | 5281343 | Appearance | Powder |
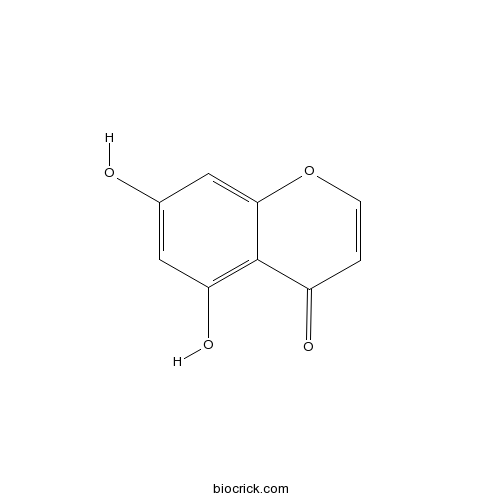
| Formula | C9H6O4 | M.Wt | 178.14 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | >7.1mg/mL in DMSO | ||
| Chemical Name | 5,7-dihydroxychromen-4-one | ||
| SMILES | C1=COC2=CC(=CC(=C2C1=O)O)O | ||
| Standard InChIKey | NYCXYKOXLNBYID-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 5,7-Dihydroxychromone is one of the active compounds that may contribute to regulate blood glucose levels. 5,7-Dihydroxychromone exerts neuroprotective effect against 6-OHDA-induced oxidative stress and apoptosis by activating Nrf2/ARE signal. |
| Targets | Nrf2 | HO-1 | PPAR | Caspase | ROS | NQO1 |
| In vitro | Neuroprotection against 6-OHDA-induced oxidative stress and apoptosis in SH-SY5Y cells by 5,7-Dihydroxychromone: Activation of the Nrf2/ARE pathway.[Pubmed: 25818191]Life Sci. 2015 Jun 1;130:25-30.The aim of this study was to prove the neuroprotective effect of 5,7-Dihydroxychromone (DHC) through the Nrf2/ARE signaling pathway. To elucidate the mechanism, we investigated whether 6-hydroxydopamine (6-OHDA)-induced neurotoxicity in SH-SY5Y cells could be attenuated by DHC via activating the Nrf2/ARE signal and whether 5,7-Dihydroxychromone could down-regulate 6-OHDA-induced excessive ROS generation |
| In vivo | Anti-diabetic properties of Daphniphyllum macropodum fruit and its active compound.[Pubmed: 25130743]Biosci Biotechnol Biochem. 2014;78(8):1392-401.Among the compounds isolated, 5,7-Dihydroxychromone potently induced the differentiation of mouse 3T3-L1 preadipocytes. DME and 5,7-Dihydroxychromone increased PPARγ and liver X receptor α (LXRα) mRNA expression levels. |

5,7-Dihydroxychromone Dilution Calculator

5,7-Dihydroxychromone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.6136 mL | 28.0678 mL | 56.1356 mL | 112.2712 mL | 140.3391 mL |
| 5 mM | 1.1227 mL | 5.6136 mL | 11.2271 mL | 22.4542 mL | 28.0678 mL |
| 10 mM | 0.5614 mL | 2.8068 mL | 5.6136 mL | 11.2271 mL | 14.0339 mL |
| 50 mM | 0.1123 mL | 0.5614 mL | 1.1227 mL | 2.2454 mL | 2.8068 mL |
| 100 mM | 0.0561 mL | 0.2807 mL | 0.5614 mL | 1.1227 mL | 1.4034 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Hesperetin 7-O-glucoside
Catalog No.:BCN5237
CAS No.:31712-49-9
- Aminophylline
Catalog No.:BCC2300
CAS No.:317-34-0
- Levomefolic acid
Catalog No.:BCC1703
CAS No.:31690-09-2
- Pinusolide
Catalog No.:BCN5236
CAS No.:31685-80-0
- Gatifloxacin mesylate
Catalog No.:BCC4225
CAS No.:316819-28-0
- 6-Aminonicotinic acid
Catalog No.:BCC8764
CAS No.:3167-49-5
- Palifosfamide
Catalog No.:BCC1833
CAS No.:31645-39-3
- 9-Anthracenylmethyl acrylate
Catalog No.:BCC8798
CAS No.:31645-34-8
- 5,7-Dihydroxy-6,8-dimethoxyflavone
Catalog No.:BCN5235
CAS No.:3162-45-6
- Artocarpesin
Catalog No.:BCN8071
CAS No.:3162-09-2
- SCH 442416
Catalog No.:BCC7372
CAS No.:316173-57-6
- Moroxydine HCl
Catalog No.:BCC4802
CAS No.:3160-91-6
- 3,5,7-Trihydroxychromone
Catalog No.:BCN7479
CAS No.:31721-95-6
- GW501516
Catalog No.:BCC2268
CAS No.:317318-70-0
- GW0742
Catalog No.:BCC2267
CAS No.:317318-84-6
- O-1602
Catalog No.:BCC7487
CAS No.:317321-41-8
- TCS 2314
Catalog No.:BCC6080
CAS No.:317353-73-4
- 2-Methyl-4-(2-methylbenzoylamino)benzoic acid
Catalog No.:BCC8579
CAS No.:317374-08-6
- 3-Deoxyaconitine
Catalog No.:BCN2797
CAS No.:3175-95-9
- (-)-Lyoniresinol
Catalog No.:BCN3488
CAS No.:31768-94-2
- H-Ala-pNA.HCl
Catalog No.:BCC3195
CAS No.:31796-55-1
- Propranolol HCl
Catalog No.:BCC4336
CAS No.:318-98-9
- H-Phe-OEt.HCl
Catalog No.:BCC3007
CAS No.:3182-93-2
- H-Phenylalaninol
Catalog No.:BCC2719
CAS No.:3182-95-4
Anti-diabetic properties of Daphniphyllum macropodum fruit and its active compound.[Pubmed:25130743]
Biosci Biotechnol Biochem. 2014;78(8):1392-401.
We evaluated in vitro anti-diabetic activities of 497 native plants of Jeju Island (South Korea) by measuring the induction of adipocyte differentiation. Among the plants, Daphniphyllum macropodum fruit extract (DME) had the highest peroxisome proliferator-activated receptor gamma (PPARgamma) agonist activity and was therefore selected as a potential source of anti-diabetic agents. To elucidate the active components of DME, constituent compounds were purified and their effects on the adipocyte differentiation were studied. Using activity-guided fractionation, four compounds were isolated from DME and their adipogenic effects were evaluated. Among the compounds isolated, 5,7-Dihydroxychromone potently induced the differentiation of mouse 3T3-L1 preadipocytes. DME and 5,7-Dihydroxychromone increased PPARgamma and liver X receptor alpha (LXRalpha) mRNA expression levels. To determine whether the adipogenic effects we observed might affect serum glucose levels, we undertook in vivo experiment using streptozotocin-/high-fat diet-induced type 2 diabetes mouse model. DME supplementation reduced serum glucose, total cholesterol, and triacylglycerol levels in diabetes mice. These results suggest that DME may be useful for the prevention and treatment of type 2 diabetes mellitus. Moreover, it was proposed that 5,7-Dihydroxychromone isolated from DME is one of the active compounds that may contribute to regulate blood glucose levels.
Neuroprotection against 6-OHDA-induced oxidative stress and apoptosis in SH-SY5Y cells by 5,7-Dihydroxychromone: Activation of the Nrf2/ARE pathway.[Pubmed:25818191]
Life Sci. 2015 Jun 1;130:25-30.
AIMS: The aim of this study was to prove the neuroprotective effect of 5,7-Dihydroxychromone (DHC) through the Nrf2/ARE signaling pathway. To elucidate the mechanism, we investigated whether 6-hydroxydopamine (6-OHDA)-induced neurotoxicity in SH-SY5Y cells could be attenuated by DHC via activating the Nrf2/ARE signal and whether DHC could down-regulate 6-OHDA-induced excessive ROS generation MAIN METHODS: To evaluate the neuroprotective effect of DHC against 6-OHDA-induced apoptosis, FACS analysis was performed using PI staining. The inhibitory effect of DHC against 6-OHDA-induced ROS generation was evaluated by DCFH-DA staining assay. Additionally, translocation of Nrf2 to the nucleus and increased Nrf2/ARE binding activity, which subsequently resulted in the up-regulation of the Nrf2-dependent antioxidant gene expressions including HO-1, NQO1, and GCLc, were evaluated by Western blotting and EMSA. KEY FINDINGS: Pre-treatment of DHC, one of the constituents of Cudrania tricuspidata, significantly protects 6-OHDA-induced neuronal cell death and ROS generation. Also, DHC inhibited the expression of activated caspase-3 and caspase-9 and cleaved PARP in 6-OHDA-induced SH-SY5Y cells. DHC induced the translocation of Nrf2 to the nucleus and increased Nrf2/ARE binding activity which results in the up-regulation of the expression of Nrf2-dependent antioxidant genes, including HO-1, NQO1, and GCLc. The addition of Nrf2 siRNA abolished the neuroprotective effect of DHC against 6-OHDA-induced neurotoxicity and the expression of Nrf2-mediated antioxidant genes. SIGNIFICANCE: Activation of Nrf2/ARE signal by DHC exerted neuroprotective effects against 6-OHDA-induced oxidative stress and apoptosis. This finding will give an insight that activating Nrf2/ARE signal could be a new potential therapeutic strategy for neurodegenerative disease.


