Gatifloxacin mesylateCAS# 316819-28-0 |
- Dexrazoxane HCl (ICRF-187, ADR-529)
Catalog No.:BCC1087
CAS No.:149003-01-0
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- Etoposide
Catalog No.:BCC1151
CAS No.:33419-42-0
- Genistein
Catalog No.:BCN5499
CAS No.:446-72-0
- Ellagic acid
Catalog No.:BCN5533
CAS No.:476-66-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 316819-28-0 | SDF | Download SDF |
| PubChem ID | 16040196 | Appearance | Powder |
| Formula | C20H26FN3O7S | M.Wt | 471.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AM 1155 mesylate; BMS 206584-01 mesylate; PD 135432 mesylate | ||
| Solubility | Soluble in DMSO | ||
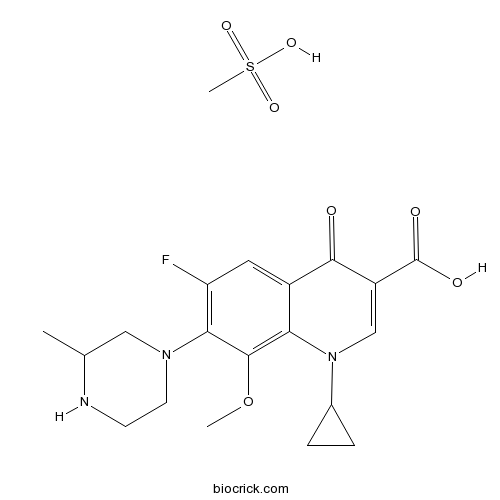
| Chemical Name | 1-cyclopropyl-6-fluoro-8-methoxy-7-(3-methylpiperazin-1-yl)-4-oxoquinoline-3-carboxylic acid;methanesulfonic acid | ||
| SMILES | CC1CN(CCN1)C2=C(C=C3C(=C2OC)N(C=C(C3=O)C(=O)O)C4CC4)F.CS(=O)(=O)O | ||
| Standard InChIKey | PMMNVFFMFJMFDB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H22FN3O4.CH4O3S/c1-10-8-22(6-5-21-10)16-14(20)7-12-15(18(16)27-2)23(11-3-4-11)9-13(17(12)24)19(25)26;1-5(2,3)4/h7,9-11,21H,3-6,8H2,1-2H3,(H,25,26);1H3,(H,2,3,4) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Gatifloxacin (mesylate) is an antibiotic of the fourth-generation fluoroquinolone family, it inhibits the bacterial enzymes DNA gyrase and topoisomerase IV.
Target: Antibacterial
Gatifloxacin (mesylate) is the mesylate salt of Gatifloxacin which is an antibiotic of the fourth-generation fluoroquinolone family, that like other members of that family, inhibits the bacterial enzymes DNA gyrase and topoisomerase IV. Gatifloxacin had activity equal to that of tosufloxacin and activity more potent than those of norfloxacin, ofloxacin, ciprofloxacin, and sparfloxacin against the second-step mutants (grlA gyrA; gatifloxacin MIC range, 1.56 to 3.13 microg/ml) and had the most potent activity against the third-step mutants (grlA gyrA grlA; gatifloxacin MIC range, 1.56 to 6.25 microg/ml), suggesting that gatifloxacin possesses the most potent inhibitory activity against singly mutated topo IV and singly mutated DNA gyrase among the quinolones tested [1].
Ophthalmic gatifloxacin 0.3% is at least as effective as ciprofloxacin at healing corneal ulcers infected with Pseudomonas aeruginosa when gatifloxacin is administered less frequently than ciprofloxacin. Trends favored gatifloxacin in fluorescein retention scores [2].
Clinical indications: Bacterial infection
Toxicity: Hepatotoxicity; Acute pancreatitis [3]; Torsades de pointes [4] References: | |||||

Gatifloxacin mesylate Dilution Calculator

Gatifloxacin mesylate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1209 mL | 10.6045 mL | 21.2089 mL | 42.4178 mL | 53.0223 mL |
| 5 mM | 0.4242 mL | 2.1209 mL | 4.2418 mL | 8.4836 mL | 10.6045 mL |
| 10 mM | 0.2121 mL | 1.0604 mL | 2.1209 mL | 4.2418 mL | 5.3022 mL |
| 50 mM | 0.0424 mL | 0.2121 mL | 0.4242 mL | 0.8484 mL | 1.0604 mL |
| 100 mM | 0.0212 mL | 0.106 mL | 0.2121 mL | 0.4242 mL | 0.5302 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Gatifloxacin (mesylate) is an antibiotic of the fourth-generation fluoroquinolone family, it inhibits the bacterial enzymes DNA gyrase and topoisomerase IV.
- 6-Aminonicotinic acid
Catalog No.:BCC8764
CAS No.:3167-49-5
- Palifosfamide
Catalog No.:BCC1833
CAS No.:31645-39-3
- 9-Anthracenylmethyl acrylate
Catalog No.:BCC8798
CAS No.:31645-34-8
- 5,7-Dihydroxy-6,8-dimethoxyflavone
Catalog No.:BCN5235
CAS No.:3162-45-6
- Artocarpesin
Catalog No.:BCN8071
CAS No.:3162-09-2
- SCH 442416
Catalog No.:BCC7372
CAS No.:316173-57-6
- Moroxydine HCl
Catalog No.:BCC4802
CAS No.:3160-91-6
- Z-Ile-OH
Catalog No.:BCC2593
CAS No.:3160-59-6
- Z-Asp(OMe)-OH
Catalog No.:BCC2790
CAS No.:3160-47-2
- Emetine dihydrochloride
Catalog No.:BCN8307
CAS No.:316-42-7
- Berberine Sulphate
Catalog No.:BCC8131
CAS No.:316-41-6
- Heraclenol
Catalog No.:BCN5234
CAS No.:31575-93-6
- Pinusolide
Catalog No.:BCN5236
CAS No.:31685-80-0
- Levomefolic acid
Catalog No.:BCC1703
CAS No.:31690-09-2
- Aminophylline
Catalog No.:BCC2300
CAS No.:317-34-0
- Hesperetin 7-O-glucoside
Catalog No.:BCN5237
CAS No.:31712-49-9
- 5,7-Dihydroxychromone
Catalog No.:BCN4652
CAS No.:31721-94-5
- 3,5,7-Trihydroxychromone
Catalog No.:BCN7479
CAS No.:31721-95-6
- GW501516
Catalog No.:BCC2268
CAS No.:317318-70-0
- GW0742
Catalog No.:BCC2267
CAS No.:317318-84-6
- O-1602
Catalog No.:BCC7487
CAS No.:317321-41-8
- TCS 2314
Catalog No.:BCC6080
CAS No.:317353-73-4
- 2-Methyl-4-(2-methylbenzoylamino)benzoic acid
Catalog No.:BCC8579
CAS No.:317374-08-6
- 3-Deoxyaconitine
Catalog No.:BCN2797
CAS No.:3175-95-9
Comprehensive evaluation of formulation factors for ocular penetration of fluoroquinolones in rabbits using cassette dosing technique.[Pubmed:26955263]
Drug Des Devel Ther. 2016 Feb 22;10:811-23.
OBJECTIVE: Corneal permeability of drugs is an important factor used to assess the efficacy of topical preparations. Transcorneal penetration of drugs from aqueous formulation is governed by various physiological, physiochemical, and formulation factors. In the present study, we investigated the effect of formulation factors like concentration, pH, and volume of instillation across the cornea using cassette dosing technique for ophthalmic fluoroquinolones (FQs). MATERIALS AND METHODS: Sterile cocktail formulations were prepared using four congeneric ophthalmic FQs (ofloxacin, sparfloxacin, pefloxacin mesylate, and gatifloxacin) at concentrations of 0.025%, 0.5%, and 0.1%. Each formulation was adjusted to different pH ranges (4.5, 7.0, and 8.0) and assessed for transcorneal penetration in vivo in rabbit's cornea (n=4 eyes) at three different volumes (12.5, 25, and 50 muL). Aqueous humor was aspirated through paracentesis after applying local anesthesia at 0, 5, 15, 30, 60, 120, and 240 minutes postdosing. The biosamples collected from a total of 27 groups were analyzed using liquid chromatography-tandem mass spectroscopy to determine transcorneal permeability of all four FQs individually. RESULTS: Increase in concentration showed an increase in penetration up to 0.05%; thereafter, the effect of concentration was found to be dependent on volume of instillation as we observed a decrease in transcorneal penetration. The highest transcorneal penetration of all FQs was observed at pH 7.0 at concentration 0.05% followed by 0.025% at pH 4.5. Lastly, increasing the volume of instillation from 12.5 to 50 muL showed a significant fall in transcorneal penetration. CONCLUSION: The study concludes that formulation factors showed discernible effect on transcorneal permeation; therefore, due emphasis should be given on drug development and design of ophthalmic formulation.


