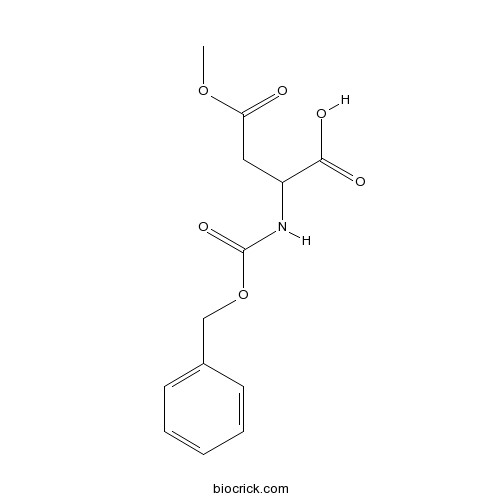
Z-Asp(OMe)-OHCAS# 3160-47-2 |
- Fulvestrant
Catalog No.:BCC1081
CAS No.:129453-61-8
- Bazedoxifene
Catalog No.:BCC1411
CAS No.:198481-32-2
- Bazedoxifene acetate
Catalog No.:BCC1412
CAS No.:198481-33-3
- (E)-2-Decenoic acid
Catalog No.:BCC1292
CAS No.:334-49-6
- Toremifene
Catalog No.:BCC2010
CAS No.:89778-26-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3160-47-2 | SDF | Download SDF |
| PubChem ID | 273302 | Appearance | Powder |
| Formula | C13H15NO6 | M.Wt | 281.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-methoxy-4-oxo-2-(phenylmethoxycarbonylamino)butanoic acid | ||
| SMILES | COC(=O)CC(C(=O)O)NC(=O)OCC1=CC=CC=C1 | ||
| Standard InChIKey | PHMBNDDHIBIDRQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H15NO6/c1-19-11(15)7-10(12(16)17)14-13(18)20-8-9-5-3-2-4-6-9/h2-6,10H,7-8H2,1H3,(H,14,18)(H,16,17) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Z-Asp(OMe)-OH Dilution Calculator

Z-Asp(OMe)-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5549 mL | 17.7746 mL | 35.5492 mL | 71.0985 mL | 88.8731 mL |
| 5 mM | 0.711 mL | 3.5549 mL | 7.1098 mL | 14.2197 mL | 17.7746 mL |
| 10 mM | 0.3555 mL | 1.7775 mL | 3.5549 mL | 7.1098 mL | 8.8873 mL |
| 50 mM | 0.0711 mL | 0.3555 mL | 0.711 mL | 1.422 mL | 1.7775 mL |
| 100 mM | 0.0355 mL | 0.1777 mL | 0.3555 mL | 0.711 mL | 0.8887 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Z-Asp(OMe)-OH
- Emetine dihydrochloride
Catalog No.:BCN8307
CAS No.:316-42-7
- Berberine Sulphate
Catalog No.:BCC8131
CAS No.:316-41-6
- Heraclenol
Catalog No.:BCN5234
CAS No.:31575-93-6
- 4EGI-1
Catalog No.:BCC5337
CAS No.:315706-13-9
- PTC-209
Catalog No.:BCC5111
CAS No.:315704-66-6
- JK 184
Catalog No.:BCC3936
CAS No.:315703-52-7
- STF-62247
Catalog No.:BCC4960
CAS No.:315702-99-9
- TC-DAPK 6
Catalog No.:BCC1989
CAS No.:315694-89-4
- Kavain
Catalog No.:BCN8295
CAS No.:3155-48-4
- 1,18-Octadecanediol
Catalog No.:BCN5233
CAS No.:3155-43-9
- Matsukaze-lactone
Catalog No.:BCN7580
CAS No.:3153-73-9
- O-Nornuciferine
Catalog No.:BCN7074
CAS No.:3153-55-7
- Z-Ile-OH
Catalog No.:BCC2593
CAS No.:3160-59-6
- Moroxydine HCl
Catalog No.:BCC4802
CAS No.:3160-91-6
- SCH 442416
Catalog No.:BCC7372
CAS No.:316173-57-6
- Artocarpesin
Catalog No.:BCN8071
CAS No.:3162-09-2
- 5,7-Dihydroxy-6,8-dimethoxyflavone
Catalog No.:BCN5235
CAS No.:3162-45-6
- 9-Anthracenylmethyl acrylate
Catalog No.:BCC8798
CAS No.:31645-34-8
- Palifosfamide
Catalog No.:BCC1833
CAS No.:31645-39-3
- 6-Aminonicotinic acid
Catalog No.:BCC8764
CAS No.:3167-49-5
- Gatifloxacin mesylate
Catalog No.:BCC4225
CAS No.:316819-28-0
- Pinusolide
Catalog No.:BCN5236
CAS No.:31685-80-0
- Levomefolic acid
Catalog No.:BCC1703
CAS No.:31690-09-2
- Aminophylline
Catalog No.:BCC2300
CAS No.:317-34-0
Synthesis of a precursor tripeptide Z-Asp-Val-Tyr-OH of thymopentin by chemo-enzymatic method.[Pubmed:23030464]
Prep Biochem Biotechnol. 2012;42(6):520-34.
The precursor tripeptide of thymopentin was synthesized by a combination of chemical and enzymatic methods. First, Val-Tyr-OH dipeptide was synthesized by a novel chemical method in two steps involving preparation of NCA-Val. Second, the linkage of the third amino acid Z-Asp-OMe to Val-Tyr-OH was completed by an enzymatic method under kinetic control. An industrial alkaline protease alcalase was used in water-organic cosolvent systems. The synthesis reaction conditions were optimized by examining the effects of several factors including organic solvents, water content, temperature, pH, and reaction time on the yield of Z-Asp-Val-Tyr-OH. The optimum condition is of pH 10.0, 35 degrees C, acetonitrile/Na(2)CO(3)-NaHCO(3) buffer system (85:15, v/v), and reaction time of 2.5 hr, which achieves tripeptide yield of more than 70%.
Synthesis, radiolabeling and biological activity of peptide oostatic hormone and its analogues.[Pubmed:9309578]
J Pept Res. 1997 Sep;50(3):153-8.
A series of Pro peptides containing the sequence of the oostatic hormone 3d and its shorter analogues 3a-3c differing in a number of the C-terminal Pro residues was prepared for a study of its effect on oogenesis in Sarcophaga bullata Parker (Diptera). Peptides 3a-3d were synthesized in solution by the fragment condensation of Boc-Tyr-Asp(OtBu)-Pro-Ala-Pro-OH (2f) with Pro oligopeptides H-(Pro)2-5-OtBu. The amino-terminal protected pentapeptide acid 2f was prepared by a stepwise procedure from TFA.H-Ala-Pro-OMe using Boc-Pro-OH, Z-Asp(OtBu)-OSu and Boc-Tyr-OSu. The H(Z)-(Pro)2-5-OtBu oligopeptides 1a-1h were synthesized from Z-Pro-OH and H-Pro-OtBu by a combination of stepwise procedure and fragment condensation. The 125I-labeled molecules of the octapeptide 3b and decapeptide 3d were used for radiotracer distribution studies. Evidence of content of the labeled peptide material in various parts of the insect body (ovaries, head, intestine) is presented. The time distribution of the labeled material in the insect organs was correlated with results of histological analysis of ovaries treated by nonlabeled peptides. The peptides assayed affected processes of egg development in 20-60% of ovarioles. The decapeptide 3d caused changes consisting in some resorbed egg chambers and normal appearance of vitellogenic eggs, whereas the octapeptide 3b caused abnormal yolk deposition and formation of big eggs with irregular yolk granules, proliferation of follicular epithelium in some egg chambers and about the same amount of resorbed egg chambers as decapeptide. These structural differences are complementary to the different values of organ radioactivities.
Influence of reaction conditions on syntheses of sweetener precursors catalyzed by thermolysin in tert-amyl alcohol.[Pubmed:9832308]
J Pept Res. 1998 Oct;52(4):300-4.
The activity of enzymes to form a peptide bond in organic solvent was greatly influenced by observed pH and water content. The precursors of two sweeteners, P-Asp-Xaa-OR (P=Z or For, Xaa-OR=Phe-OMe or Ala-OcHex), were synthesized by enzyme, and the reaction conditions were studied systematically. Z-Asp-OH was coupled with H-Phe-OMe or H-Ala-OcHex by thermolysin in tert-amyl alcohol. The best coupling results were obtained when the optimized observed pH was 8 or 9, and the water content was about 6% (V/V). The protecting group Z is better than For under the reaction conditions and H-Phe-OMe is a better nucleophile than H-Ala-OcHex. The expected optically pure peptides were obtained when the racemic amino acids were used as amino components in the starting materials. The physical constants of P-Asp-Xaa-OR synthesized by thermolysin are identical with those of peptides synthesized by chemical method.


