JK 184Hh signaling inhibitor CAS# 315703-52-7 |
- Cyclopamine
Catalog No.:BCN2964
CAS No.:4449-51-8
- Purmorphamine
Catalog No.:BCC3641
CAS No.:483367-10-8
- GANT61
Catalog No.:BCC1090
CAS No.:500579-04-4
- GANT 58
Catalog No.:BCC1587
CAS No.:64048-12-0
- GDC-0449 (Vismodegib)
Catalog No.:BCC1285
CAS No.:879085-55-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 315703-52-7 | SDF | Download SDF |
| PubChem ID | 1069686 | Appearance | Powder |
| Formula | C19H18N4OS | M.Wt | 350.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (142.68 mM) *"≥" means soluble, but saturation unknown. | ||
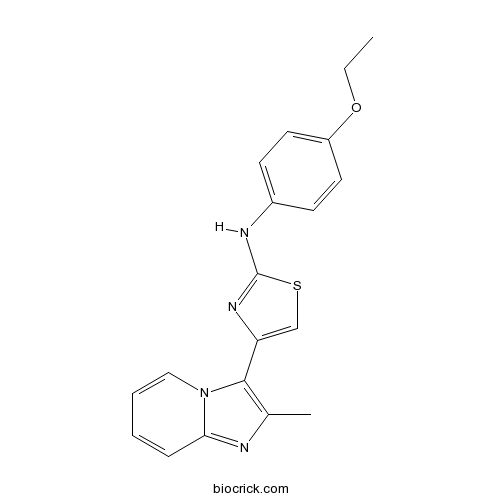
| Chemical Name | N-(4-ethoxyphenyl)-4-(2-methylimidazo[1,2-a]pyridin-3-yl)-1,3-thiazol-2-amine | ||
| SMILES | CCOC1=CC=C(C=C1)NC2=NC(=CS2)C3=C(N=C4N3C=CC=C4)C | ||
| Standard InChIKey | ROYXIPOUVGDTAO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H18N4OS/c1-3-24-15-9-7-14(8-10-15)21-19-22-16(12-25-19)18-13(2)20-17-6-4-5-11-23(17)18/h4-12H,3H2,1-2H3,(H,21,22) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent downstream hedgehog (Hh) signaling inhibitor that prevents Gli-dependent transcriptional activity (IC50 = 30 nM). Exhibits antiproliferative activity in a range of cancer cell lines (GI50 = 3 - 21 nM) and in human xenografts in vivo. Inhibits alcohol dehydrogenase 7 (Adh7) (IC50 = 210 nM) and acts as a microtubule depolymerizing agent in vitro. |

JK 184 Dilution Calculator

JK 184 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8536 mL | 14.2678 mL | 28.5356 mL | 57.0711 mL | 71.3389 mL |
| 5 mM | 0.5707 mL | 2.8536 mL | 5.7071 mL | 11.4142 mL | 14.2678 mL |
| 10 mM | 0.2854 mL | 1.4268 mL | 2.8536 mL | 5.7071 mL | 7.1339 mL |
| 50 mM | 0.0571 mL | 0.2854 mL | 0.5707 mL | 1.1414 mL | 1.4268 mL |
| 100 mM | 0.0285 mL | 0.1427 mL | 0.2854 mL | 0.5707 mL | 0.7134 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Potent downstream hedgehog (Hh) signaling inhibitor that prevents Gli-dependent transcriptional activity (IC50 = 30 nM). Exhibits antiproliferative activity in a range of cancer cell lines (GI50 = 3 - 21 nM) and in human xenografts in vivo. Inhibits alcoh
- STF-62247
Catalog No.:BCC4960
CAS No.:315702-99-9
- TC-DAPK 6
Catalog No.:BCC1989
CAS No.:315694-89-4
- Kavain
Catalog No.:BCN8295
CAS No.:3155-48-4
- 1,18-Octadecanediol
Catalog No.:BCN5233
CAS No.:3155-43-9
- Matsukaze-lactone
Catalog No.:BCN7580
CAS No.:3153-73-9
- O-Nornuciferine
Catalog No.:BCN7074
CAS No.:3153-55-7
- 5-Hydroxyseselin
Catalog No.:BCN3428
CAS No.:31525-75-4
- Isobavachin
Catalog No.:BCN5232
CAS No.:31524-62-6
- Sutherlandin trans-p-coumarate
Catalog No.:BCN5231
CAS No.:315236-68-1
- Ifflaiamine
Catalog No.:BCN7061
CAS No.:31520-95-3
- PAC-1
Catalog No.:BCC3600
CAS No.:315183-21-2
- Acetylheliosupine
Catalog No.:BCN1981
CAS No.:31514-30-4
- PTC-209
Catalog No.:BCC5111
CAS No.:315704-66-6
- 4EGI-1
Catalog No.:BCC5337
CAS No.:315706-13-9
- Heraclenol
Catalog No.:BCN5234
CAS No.:31575-93-6
- Berberine Sulphate
Catalog No.:BCC8131
CAS No.:316-41-6
- Emetine dihydrochloride
Catalog No.:BCN8307
CAS No.:316-42-7
- Z-Asp(OMe)-OH
Catalog No.:BCC2790
CAS No.:3160-47-2
- Z-Ile-OH
Catalog No.:BCC2593
CAS No.:3160-59-6
- Moroxydine HCl
Catalog No.:BCC4802
CAS No.:3160-91-6
- SCH 442416
Catalog No.:BCC7372
CAS No.:316173-57-6
- Artocarpesin
Catalog No.:BCN8071
CAS No.:3162-09-2
- 5,7-Dihydroxy-6,8-dimethoxyflavone
Catalog No.:BCN5235
CAS No.:3162-45-6
- 9-Anthracenylmethyl acrylate
Catalog No.:BCC8798
CAS No.:31645-34-8
Predictors of Indoor Radon Concentrations in Pennsylvania, 1989-2013.[Pubmed:25856050]
Environ Health Perspect. 2015 Nov;123(11):1130-7.
BACKGROUND: Radon is the second-leading cause of lung cancer worldwide. Most indoor exposure occurs by diffusion of soil gas. Radon is also found in well water, natural gas, and ambient air. Pennsylvania has high indoor radon concentrations; buildings are often tested during real estate transactions, with results reported to the Department of Environmental Protection (PADEP). OBJECTIVES: We evaluated predictors of indoor radon concentrations. METHODS: Using first-floor and basement indoor radon results reported to the PADEP between 1987 and 2013, we evaluated associations of radon concentrations (natural log transformed) with geology, water source, building characteristics, season, weather, community socioeconomic status, community type, and unconventional natural gas development measures based on drilled and producing wells. RESULTS: Primary analysis included 866,735 first measurements by building, with the large majority from homes. The geologic rock layer on which the building sat was strongly associated with radon concentration (e.g., Axemann Formation, median = 365 Bq/m3, IQR = 167-679 vs. Stockton Formation, median = 93 Bq/m3, IQR = 52-178). In adjusted analysis, buildings using well water had 21% higher concentrations (beta = 0.191, 95% CI: 0.184, 0.198). Buildings in cities (vs. townships) had lower concentrations (beta = -0.323, 95% CI: -0.333, -0.314). When we included multiple tests per building, concentrations declined with repeated measurements over time. Between 2005 and 2013, 7,469 unconventional wells were drilled in Pennsylvania. Basement radon concentrations fluctuated between 1987 and 2003, but began an upward trend from 2004 to 2012 in all county categories (p < 0.001), with higher levels in counties having >/= 100 drilled wells versus counties with none, and with highest levels in the Reading Prong. CONCLUSIONS: Geologic unit, well water, community, weather, and unconventional natural gas development were associated with indoor radon concentrations. Future studies should include direct environmental measurement of radon, as well as building features unavailable for this analysis. CITATION: Casey JA, Ogburn EL, Rasmussen SG, Irving JK, Pollak J, Locke PA, Schwartz BS. 2015. Predictors of indoor radon concentrations in Pennsylvania, 1989-2013. Environ Health Perspect 123:1130-1137; http://dx.doi.org/10.1289/ehp.1409014.
Hepatitis B Virus Infection Is a Risk Factor for Periprosthetic Joint Infection Among Males After Total Knee Arthroplasty: A Taiwanese Nationwide Population-Based Study.[Pubmed:27258517]
Medicine (Baltimore). 2016 May;95(22):e3806.
Periprosthetic joint infection (PJI) is a grave complication that can affect patients undergoing total knee arthroplasty (TKA). In this study, we aim to determine whether hepatitis B virus (HBV) infection is a risk factor for PJIs.All patients (1184 males, 3435 females) undergoing primary TKA in Taiwan from 2001 to 2010 were recruited for analysis.The incidence of PJI was 523 among the males with HBV infection and 110 among the males without HBV (per 10,000 person-years, P < 0.001). The males with HBV infection had a 4.32-fold risk of PJI compared with the males without HBV. HBV infection and diabetes were the risk factors for PJI among males. The incidence of PJI was 58.8 among the females with HBV infection and 75.2 among the females without HBV (per 10,000 person-years, P = 0.67). The risk of PJI was higher for the males with HBV infection than for the males without 0.5 to 1 year after TKA (hazard ratio [HR] = 18.7, 95% confidence interval (CI) = 1.90-184) and >1 year after TKA (HR = 4.80, 95% CI = 1.57-14.7).HBV infection is a risk factor for PJI after TKA among males.
Structural and photophysical properties of HPPCO (4-hydroxy-5-phenyl-6H-pyrido[3,2,1-jk]carbazol-6-one) derivatives.[Pubmed:25011045]
Spectrochim Acta A Mol Biomol Spectrosc. 2015 Jan 5;134:184-90.
Proton-substitution effects of 4-hydroxy-5-phenyl-6H-pyrido[3,2,1-jk]carbazol-6-one (HPPCO) on structural and photophysical properties were presented. HPPCO crystallized in the orthorhombic space group Pbca with an intermolecular hydrogen bonding between OH and oxygen atom of the carbonyl. The proton-substituted derivatives, 6-oxo-5-phenyl-6H-pyrido[3,2,1-jk]carbazol-4-yl acetate (OPPCA) and 6-oxo-5-phenyl-6H-pyrido[3,2,1-jk]carbazol-4-yl benzoate (OPPCB), crystallized in the monoclinic P2(1)/c space group. For OPPCA and OPPCB, a weak interaction between carbonyl oxygen atom in the substituted group and carbon atom in the fused ring was responsible for three-dimensional arrangements. In addition, 6-oxo-5-phenyl-6H-pyrido[3,2,1-jk]carbazol-4-yl furan-2-carboxylate (OPPCF), and 6-oxo-5-phenyl-6H-pyrido[3,2,1-jk]carbazol-4-yl naphthoate (OPPCN) were also synthesized. HPPCO and the four derivatives excited by ultraviolet (UV) light produced blue emission. Proton substitution of the OH group significantly increased the radiative transitions and moderately decreased the non-radiative transitions. Consequently the luminescence quantum yields of the derivatives enhanced more than 4.6-fold, no matter what the groups were substituted. Structural and optical properties were further determined using density functional theory (DFT) and ZINDO calculations. The planar structure of the pyridocarbazole-fused ring resulted in pi-->pi(*) electronic transitions within the main frame, with an additional transition from the n(O) of carbonyl to the pi(*) of the main frame. The three excited states that arose from these transitions were responsible for the blue luminescence.
Nitric oxide is involved in Mycobacterium bovis bacillus Calmette-Guerin-activated Jagged1 and Notch1 signaling.[Pubmed:20147635]
J Immunol. 2010 Mar 15;184(6):3117-26.
Pathogenic mycobacteria have evolved unique strategies to survive within the hostile environment of macrophages. Modulation of key signaling cascades by NO, generated by the host during infection, assumes critical importance in overall cell-fate decisions. We show that NO is a critical factor in Mycobacterium bovis bacillus Calmette-Guerin-mediated Notch1 activation, as the generation of activated Notch1 or expression of Notch1 target genes matrix metalloproteinase-9 (MMP-9) or Hes1 was abrogated in macrophages derived from inducible NO synthase (iNOS) knockout (iNOS(-/-)), but not from wild-type, mice. Interestingly, expression of the Notch1 ligand Jagged1 was compromised in M. bovis bacillus Calmette-Guerin-stimulated iNOS(-/-) macrophages, and loss of Jagged1 expression or Notch1 signaling could be rescued by NO donors. Signaling perturbations or genetic approaches implicated that robust expression of MMP-9 or Hes1 required synergy and cross talk between TLR2 and canonical Notch1-PI3K cascade. Further, CSL/RBP-Jk contributed to TLR2-mediated expression of MMP-9 or Hes1. Correlative evidence shows that, in a murine model for CNS tuberculosis, this mechanism operates in vivo only in brains derived from WT but not from iNOS(-/-) mice. Importantly, we demonstrate the activation of Notch1 signaling in vivo in granulomatous lesions in the brains of Mycobacterium tuberculosis-infected human patients with tuberculous meningitis. Current investigation identifies NO as a pathological link that modulates direct cooperation of TLR2 with Notch1-PI3K signaling or Jagged1 to regulate specific components of TLR2 responses. These findings provide new insights into mechanisms by which Notch1, TLR2, and NO signals are integrated in a cross talk that modulates a defined set of effector functions in macrophages.
Sequential Immunization with gp140 Boosts Immune Responses Primed by Modified Vaccinia Ankara or DNA in HIV-Uninfected South African Participants.[Pubmed:27583368]
PLoS One. 2016 Sep 1;11(9):e0161753.
BACKGROUND: The safety and immunogenicity of SAAVI DNA-C2 (4 mg IM), SAAVI MVA-C (2.9 x 109 pfu IM) and Novartis V2-deleted subtype C gp140 (100 mcg) with MF59 adjuvant in various vaccination regimens was evaluated in HIV-uninfected adults in South Africa. METHODS: Participants at three South African sites were randomized (1:1:1:1) to one of four vaccine regimens: MVA prime, sequential gp140 protein boost (M/M/P/P); concurrent MVA/gp140 (MP/MP); DNA prime, sequential MVA boost (D/D/M/M); DNA prime, concurrent MVA/gp140 boost (D/D/MP/MP) or placebo. Peak HIV specific humoral and cellular responses were measured. RESULTS: 184 participants were enrolled: 52% were female, all were Black/African, median age was 23 years (range, 18-42 years) and 79% completed all vaccinations. 159 participants reported at least one adverse event, 92.5% were mild or moderate. Five, unrelated, serious adverse events were reported. The M/M/P/P and D/D/MP/MP regimens induced the strongest peak neutralizing and binding antibody responses and the greatest CD4+ T-cell responses to Env. All peak neutralizing and binding antibody responses decayed with time. The MVA, but not DNA, prime contributed to the humoral and cellular immune responses. The D/D/M/M regimen was poorly immunogenic overall but did induce modest CD4+ T-cell responses to Gag and Pol. CD8+ T-cell responses to any antigen were low for all regimens. CONCLUSIONS: The SAAVI DNA-C2, SAAVI MVA-C and Novartis gp140 with MF59 adjuvant in various combinations were safe and induced neutralizing and binding antibodies and cellular immune responses. Sequential immunization with gp140 boosted immune responses primed by MVA or DNA. The best overall immune responses were seen with the M/M/P/P regimen. TRIAL REGISTRATION: ClinicalTrials.gov NCT01418235.
The imidazopyridine derivative JK184 reveals dual roles for microtubules in Hedgehog signaling.[Pubmed:19222062]
Angew Chem Int Ed Engl. 2009;48(13):2321-4.
Eradicating hedgehogs: The title molecule has been previously identified as a potent inhibitor of the Hedgehog signaling pathway, which gives embryonic cells information needed to develop properly. This molecule is shown to modulate Hedgehog target gene expression by depolymerizing microtubules, thus revealing dual roles of the cytoskeleton in pathway regulation (see figure).


