STF-62247Autophagy inducer in renal cell CAS# 315702-99-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 315702-99-9 | SDF | Download SDF |
| PubChem ID | 704473 | Appearance | Powder |
| Formula | C15H13N3S | M.Wt | 267.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | STF62247; STF 62247 | ||
| Solubility | DMSO : ≥ 31 mg/mL (115.95 mM) *"≥" means soluble, but saturation unknown. | ||
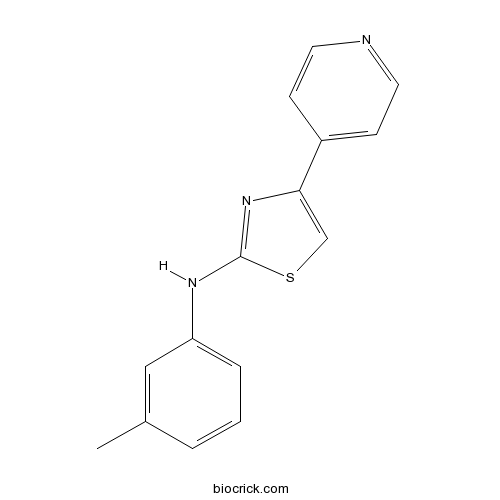
| Chemical Name | N-(3-methylphenyl)-4-pyridin-4-yl-1,3-thiazol-2-amine | ||
| SMILES | CC1=CC(=CC=C1)NC2=NC(=CS2)C3=CC=NC=C3 | ||
| Standard InChIKey | KATNUHQNJGNLPW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H13N3S/c1-11-3-2-4-13(9-11)17-15-18-14(10-19-15)12-5-7-16-8-6-12/h2-10H,1H3,(H,17,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | STF-62247 is TGN inhibitor with IC50 of 0.625μM and 16μM in RCC4 and RCC4/VHL cells,respectively.It specifically induces autophagic cell death in cells that have lost VHL, an essential mutation in the development of RCC.
IC50: 0.625/16μM in RCC4 and RCC4/VHL cells,respectively.[1]
In vitro: STF-62247 induces cytotoxicity in VHL-deficient cells in a HIF-independent manner, STF-62247 increases acidification in VHL-deficient cells ,TGN is a target of STF-62247 and a drug-selective pathway synthetically lethal in VHL-deficient cells.[1] Golgi trafficking are required as initial signals in STF-62247-induced autophagy.[2]STF-62247 increases radiosensitivity in a VHL-dependent manner.[3]
In vivo: SN12C, SN12C-VHL shRNA, or 786-O cells were implanted subcutaneously into the flanks of immunodeficient mice. The selective cytotoxicity of STF-62247 for the VHL-deficient cells was also demonstrated in 786-O cells compared to their wild-type VHL counterparts by clonogenic assay in vitro. Daily treatment with STF-62247 significantly reduced tumor growth of VHL-deficient cells. This decrease in tumor growth was concentration dependent. Importantly, drug treatment did not have any effect on the growth of SN12C tumor cells that have wild-type VHL. Together,STF-62247 reduces tumor growth in VHL-deficient cells in mice.[1] References: | |||||

STF-62247 Dilution Calculator

STF-62247 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7404 mL | 18.7021 mL | 37.4042 mL | 74.8083 mL | 93.5104 mL |
| 5 mM | 0.7481 mL | 3.7404 mL | 7.4808 mL | 14.9617 mL | 18.7021 mL |
| 10 mM | 0.374 mL | 1.8702 mL | 3.7404 mL | 7.4808 mL | 9.351 mL |
| 50 mM | 0.0748 mL | 0.374 mL | 0.7481 mL | 1.4962 mL | 1.8702 mL |
| 100 mM | 0.0374 mL | 0.187 mL | 0.374 mL | 0.7481 mL | 0.9351 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: STF-62247 inhibits tumor growth in wild-type VHL and VHL-deficient renal cell carcinoma (RCC) in a HIF-independent manner with IC50 of 16 μM and 0.625 μM, respectively.
Inactivation of the von Hippel-Lindau (VHL) tumor inhibitor gene results in a large number of renal cell carcinomas (RCCs) and is closely linked to a high degree of vascularization and poor prognosis. STF-62247 is reported to exhibit selectively cytotoxic toward VHL-deficient cells in vitro and in vivo. [1]
In vitro: In vitro study demonstrated that STF-62247 exhibited selectively cytotoxicity and tumor growth inhibitory activity towards wild-type VHL and VHL-deficient renal cell carcinoma (RCC) in a HIF-independent manner with IC50 of 16 μM and 0.625 μM, respectively. In addion, STF-62247 also resulted in cell apoptosis by inducing acidification and increasing autophagy in VHL-deficient cells. [1]
In vivo: Animal experiments for STF-62247 activity were performed according to institutional and national guidelines and approved by Stanford University's Administrative Panel on Laboratory Animal Care. Based on an in vivo mouse model, it was found that intraperitoneal injection of STF-62247 at a dose of 8 mg/kg significantly inhibited tumor growth of VHL-deficient SN12C tumor cells. [1]
Clinical trial: So far, no clinical trial has been conducted.
Reference:
[1]Turcotte S, Chan DA, Sutphin PD, Hay MP, Denny WA and Giaccia AJ. A molecule targeting vhl-deficient renal cell carcinoma that induces autophagy. Cancer Cell. 2008 Jul; 14: 90-102.
- TC-DAPK 6
Catalog No.:BCC1989
CAS No.:315694-89-4
- Kavain
Catalog No.:BCN8295
CAS No.:3155-48-4
- 1,18-Octadecanediol
Catalog No.:BCN5233
CAS No.:3155-43-9
- Matsukaze-lactone
Catalog No.:BCN7580
CAS No.:3153-73-9
- O-Nornuciferine
Catalog No.:BCN7074
CAS No.:3153-55-7
- 5-Hydroxyseselin
Catalog No.:BCN3428
CAS No.:31525-75-4
- Isobavachin
Catalog No.:BCN5232
CAS No.:31524-62-6
- Sutherlandin trans-p-coumarate
Catalog No.:BCN5231
CAS No.:315236-68-1
- Ifflaiamine
Catalog No.:BCN7061
CAS No.:31520-95-3
- PAC-1
Catalog No.:BCC3600
CAS No.:315183-21-2
- Acetylheliosupine
Catalog No.:BCN1981
CAS No.:31514-30-4
- Testosterone enanthate
Catalog No.:BCC9169
CAS No.:315-37-7
- JK 184
Catalog No.:BCC3936
CAS No.:315703-52-7
- PTC-209
Catalog No.:BCC5111
CAS No.:315704-66-6
- 4EGI-1
Catalog No.:BCC5337
CAS No.:315706-13-9
- Heraclenol
Catalog No.:BCN5234
CAS No.:31575-93-6
- Berberine Sulphate
Catalog No.:BCC8131
CAS No.:316-41-6
- Emetine dihydrochloride
Catalog No.:BCN8307
CAS No.:316-42-7
- Z-Asp(OMe)-OH
Catalog No.:BCC2790
CAS No.:3160-47-2
- Z-Ile-OH
Catalog No.:BCC2593
CAS No.:3160-59-6
- Moroxydine HCl
Catalog No.:BCC4802
CAS No.:3160-91-6
- SCH 442416
Catalog No.:BCC7372
CAS No.:316173-57-6
- Artocarpesin
Catalog No.:BCN8071
CAS No.:3162-09-2
- 5,7-Dihydroxy-6,8-dimethoxyflavone
Catalog No.:BCN5235
CAS No.:3162-45-6
STF-62247 accumulates in lysosomes and blocks late stages of autophagy to selectively target VHL-inactivated cells.[Pubmed:30758995]
Am J Physiol Cell Physiol. 2019 Feb 13.
Autophagy is a highly conserved, homeostatic process by which cytosolic components reach lysosomes for degradation. The roles that play different autophagic processes in cancer are complex and remain cancer-type and -stage dependent. Renal cell carcinoma (RCC) is the most common subtype of kidney cancer and is characterized by the inactivation of the VHL tumor suppressor. Our previous study identified a small compound, STF-62247 as an autophagy-modulating molecule causing selective cytotoxicity for VHL-inactivated cells. This present study investigates the effects of STF-62247 specifically on the macroautophagic flux to better characterize its mechanism of action in RCC. Our results clearly demonstrate that this compound is a potent blocker of late stages of autophagy. We show that inhibiting autophagy by CRISPR knockouts of autophagy-related genes rendered VHL-deficient cells insensitive to STF-62247 uncovering the importance of the autophagic pathway in STF-selective cell death. By exploiting the auto-fluorescence of STF-62247, we pinpointed its cellular localization to lysosomes. Finally, in response to prolonged STF treatments, we show that VHL-proficient cells are able to surmount the block in late stages of autophagy by restoring their lysosome numbers. Conversely, an increase in autophagic vesicles accompanied by a time-dependent decrease in lysosomes was observed in VHL-deficient cells. This is the first mechanistic study investigating STF-62447's effects on the autophagic flux in RCC. Importantly, our study re-classifies STF-62247 as a blocker of later stages of autophagy and highlights the possibility of blocking this process through lysosome disruption in VHL-mutated RCCs.
Loperamide, pimozide, and STF-62247 trigger autophagy-dependent cell death in glioblastoma cells.[Pubmed:30250198]
Cell Death Dis. 2018 Sep 24;9(10):994.
Autophagy is a well-described degradation mechanism that promotes cell survival upon nutrient starvation and other forms of cellular stresses. In addition, there is growing evidence showing that autophagy can exert a lethal function via autophagic cell death (ACD). As ACD has been implicated in apoptosis-resistant glioblastoma (GBM), there is a high medical need for identifying novel ACD-inducing drugs. Therefore, we screened a library containing 70 autophagy-inducing compounds to induce ATG5-dependent cell death in human MZ-54 GBM cells. Here, we identified three compounds, i.e. loperamide, pimozide, and STF-62247 that significantly induce cell death in several GBM cell lines compared to CRISPR/Cas9-generated ATG5- or ATG7-deficient cells, pointing to a death-promoting role of autophagy. Further cell death analyses conducted using pharmacological inhibitors revealed that apoptosis, ferroptosis, and necroptosis only play minor roles in loperamide-, pimozide- or STF-62247-induced cell death. Intriguingly, these three compounds induce massive lipidation of the autophagy marker protein LC3B as well as the formation of LC3B puncta, which are characteristic of autophagy. Furthermore, loperamide, pimozide, and STF-62247 enhance the autophagic flux in parental MZ-54 cells, but not in ATG5 or ATG7 knockout (KO) MZ-54 cells. In addition, loperamide- and pimozide-treated cells display a massive formation of autophagosomes and autolysosomes at the ultrastructural level. Finally, stimulation of autophagy by all three compounds is accompanied by dephosphorylation of mammalian target of rapamycin complex 1 (mTORC1), a well-known negative regulator of autophagy. In summary, our results indicate that loperamide, pimozide, and STF-62247 induce ATG5- and ATG7-dependent cell death in GBM cells, which is preceded by a massive induction of autophagy. These findings emphasize the lethal function and potential clinical relevance of hyperactivated autophagy in GBM.
The small molecule STF-62247 induces apoptotic and autophagic cell death in leukemic cells.[Pubmed:29963226]
Oncotarget. 2018 Jun 12;9(45):27645-27655.
Adult T cell leukemia/lymphoma (ATL) is an aggressive malignant T cell disease caused by human T cell leukemia virus-I (HTLV-1). Treatment outcomes for aggressive subtypes of ATL remain poor, with little improvement in overall survival since HTLV-1 was discovered. Therefore, new therapeutic strategies for ATL are required. STF-62247 induces autophagy and selectively kills renal cell carcinoma without apoptotic cell death. Here, we demonstrate that STF-62247 reduced cell viability and resulted in autophagosome accumulation and autophagy in leukemic cell lines (S1T, MT-2, and Jurkat). Interestingly, STF-62247 induced apoptosis in HTLV-1-infected cell lines (S1T and MT-2), as indicated by DNA fragmentation and caspase activation, but not in non-HTLV-1-infected Jurkat cells; a caspase inhibitor did not prevent this caspase-associated cell death. STF-62247 also increased nuclear endonuclease G levels. Furthermore, STF-62247 reduced cell viability and increased the number of apoptotic cells in peripheral blood mononuclear cells collected from patients with acute ATL, which has a poor prognosis. Therefore, STF-62247 may have novel therapeutic potential for ATL. This is the first evidence to demonstrate the cell growth-inhibitory effect of an autophagy inducer by caspase-dependent apoptosis and caspase-independent cell death via autophagy and endonuclease G in leukemic cells.
Quantitative proteomics to study a small molecule targeting the loss of von Hippel-Lindau in renal cell carcinomas.[Pubmed:28486780]
Int J Cancer. 2017 Aug 15;141(4):778-790.
Inactivation of the tumor suppressor gene, von Hippel-Lindau (VHL), is known to play an important role in the development of sporadic clear cell renal cell carcinomas (ccRCCs). Even if available targeted therapies for metastatic RCCs (mRCCs) have helped to improve progression-free survival rates, they have no durable clinical response. We have previously shown the feasibility of specifically targeting the loss of VHL with the identification of a small molecule, STF-62247. Understanding its functionality is crucial for developing durable personalized therapeutic agents differing from those available targeting hypoxia inducible factor (HIF-) pathways. By using SILAC proteomics, we identified 755 deregulated proteins in response to STF-62247 that were further analyzed by ingenuity pathway analysis (IPA). Bioinformatics analyses predicted alterations in 37 signaling pathways in VHL-null cells in response to treatment. Validation of some altered pathways shows that STF-62247's selectivity is linked to an important inhibition of mTORC1 activation in VHL-null cells leading to protein synthesis arrest, a mechanism differing from two allosteric inhibitors Rapamycin and Everolimus. Altogether, our study identified signaling cascades driving STF-62247 response and brings further knowledge for this molecule that shows selectivity for the loss of VHL. The use of a global SILAC approach was successful in identifying novel affected signaling pathways that could be exploited for the development of new personalized therapeutic strategies to target VHL-inactivated RCCs.
The autophagy-related protein LC3 is processed in stallion spermatozoa during short-and long-term storage and the related stressful conditions.[Pubmed:26932581]
Animal. 2016 Jul;10(7):1182-91.
Use of cooled and frozen semen is becoming increasingly prevalent in the equine industry. However, these procedures cause harmful effects in the sperm cell resulting in reduced cell lifespan and fertility rates. Apoptosis and necrosis-related events are increased during semen cryopreservation. However, a third type of cell death, named autophagy, has not been studied during equine semen storage. Light chain (LC)3 protein is a key component of the autophagy pathway. Under autophagy activation, LC3-I is lipidated and converted to LC3-II. The ratio of LC3-II/LC3-I is widely used as a marker of autophagy activation. The main objective of this study was to investigate whether LC3 is processed during cooling, freezing and the stressful conditions associated with these technologies. A secondary objective was to determine if LC3 processing can be modulated and if that may improve the quality of cryopreserved semen. LC3 processing was studied by Western blot with a specific antibody that recognized both LC3-I and LC3-II. Viability was assessed by flow cytometry. Modulation of LC3-I to LC3-II was studied with known autophagy activators (STF-62247 and rapamycin) or inhibitors (chloroquine and 3-MA) used in somatic cells. The results showed that conversion of LC3-I to LC3-II increased significantly during cooling at 4 degrees C, freezing/thawing and each of the stressful conditions tested (UV radiation, oxidative stress, osmotic stress and changes in temperature). STF-62247 and rapamycin increased the LC3-II/LC3-I ratio and decreased the viability of equine sperm, whereas chloroquine and 3-MA inhibited LC3 processing and maintained the percentage of viable cells after 2 h of incubation at 37 degrees C. Finally, refrigeration at 4 degrees C for 96 h and freezing at -196 degrees C in the presence of chloroquine and 3-MA resulted in higher percentages of viable cells. In conclusion, results showed that an 'autophagy-like' mechanism may be involved in the regulation of sperm viability during equine semen cryopreservation. Modulation of autophagy during these reproductive technologies may result in an improvement of semen quality and therefore in higher fertility rates.
Radiosensitization of renal cell carcinoma in vitro through the induction of autophagy.[Pubmed:22551566]
Radiother Oncol. 2012 Jun;103(3):388-93.
BACKGROUND AND PURPOSE: For patients diagnosed with advanced renal cell carcinoma (RCC), there are few therapeutic options. Radiation therapy is predominantly used to treat metastasis and has not proven effective in the adjuvant setting for renal cancer. Furthermore, RCC is resistant to standard cytotoxic chemotherapies. Targeted anti-angiogenics are the standard of care for RCC but are not curative. Newer agents, such as mTOR inhibitors and others that induce autophagy, have shown great promise for treating RCC. Here, we investigate the potential use of the small molecule STF-62247 to modulate radiation. MATERIALS AND METHODS: Using RCC cell lines, we evaluate sensitivity to radiation in addition to agents that induce autophagic cell death by clonogenic survival assays. Furthermore, these were also tested under physiological oxygen levels. RESULTS: STF-62247 specifically induces autophagic cell death in cells that have lost VHL, an essential mutation in the development of RCC. Treatment with STF-62247 did not alter cell cycle progression but when combined with radiation increased cell killing under oxic and hypoxic/physiological conditions. CONCLUSIONS: This study highlights the possibility of combining targeted therapeutics such as STF-62247 or temsirolimus with radiation to reduce the reliance on partial or full nephrectomy and improve patient prognosis.
Targeting cancer cells by synthetic lethality: autophagy and VHL in cancer therapeutics.[Pubmed:18818511]
Cell Cycle. 2008 Oct;7(19):2987-90.
Standard cytotoxic agents for treating cancer were developed based on their effectiveness to kill rapidly dividing cells, not on their ability to selectively kill cancer cells and spare normal tissue. Much of contemporary cancer research is aimed at identifying specific molecular features of cancers to directly target tumor cells with the hope of reducing or eliminating unwanted side effects. Targeted therapy for the treatment of cancer can be divided into two main categories: monoclonal antibodies and small molecules. In this Perspective, we review the approach of synthetic lethality to target cancer, specifically renal cell carcinoma. The concept of synthetic lethality is used to describe a genetic interaction of two non-allelic and non-lethal genes that when mutated simultaneously results in cell death. Recently, we identified a compound, STF-62247, that functions in a synthetic lethal manner to the loss of VHL, a mutation found in the majority of renal cell carcinomas.
Targeted therapy for the loss of von Hippel-Lindau in renal cell carcinoma: a novel molecule that induces autophagic cell death.[Pubmed:18769110]
Autophagy. 2008 Oct;4(7):944-6. Epub 2008 Oct 13.
Radiation and conventional cytotoxic chemotherapies are ineffective in treating renal cancer. Approximately 75 percent of renal cell carcinoma (RCC) is associated with an inactivation of the tumor suppressor gene von Hippel-Lindau (VHL). We exploited the possibility of targeting VHL-deficient RCC through synthetic lethality using a high-throughput screening approach. In this screen, STF-62247 was identified to be selectively toxic and growth inhibitory to renal cells lacking VHL. We recently demonstrated that the cytotoxicity of STF-62247 is due to dysregulated autophagy. Furthermore, the reduction of protein levels of essential autophagy pathway components such as Atg5, Atg7 and Atg9 reduces sensitivity of VHL-deficient cells to killing by STF-62247. Loss of proteins involved in Golgi trafficking sensitized RCC with wild-type VHL to killing by STF-62247, indicating a potential role for these proteins as a target of the compound. Our study supports the concept of using synthetic lethality to selectively kill VHL-deficient cells that represents a new type of targeted therapy for the treatment of RCC.
A molecule targeting VHL-deficient renal cell carcinoma that induces autophagy.[Pubmed:18598947]
Cancer Cell. 2008 Jul 8;14(1):90-102.
Renal cell carcinomas (RCCs) are refractory to standard therapies. The von Hippel-Lindau (VHL) tumor suppressor gene is inactivated in 75% of RCCs. By screening for small molecules selectively targeting VHL-deficient RCC cells, we identified STF-62247. STF-62247 induces cytotoxicity and reduces tumor growth of VHL-deficient RCC cells compared to genetically matched cells with wild-type VHL. STF-62247-stimulated toxicity occurs in a HIF-independent manner through autophagy. Reduction of protein levels of essential autophagy pathway components reduces sensitivity of VHL-deficient cells to STF-62247. Using a yeast deletion pool, we show that loss of proteins involved in Golgi trafficking increases killing by STF-62247. Thus, we have found a small molecule that selectively induces cell death in VHL-deficient cells, representing a paradigm shift for targeted therapy.


