Rosiglitazone maleatePPARγ agonist,high-affinity and selective,potent insulin sensitizer CAS# 155141-29-0 |
- BS-181
Catalog No.:BCC1439
CAS No.:1092443-52-1
- (R)-DRF053 dihydrochloride
Catalog No.:BCC7726
CAS No.:1241675-76-2
- Flavopiridol hydrochloride
Catalog No.:BCC3925
CAS No.:131740-09-5
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- PHA-848125
Catalog No.:BCC3839
CAS No.:802539-81-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 155141-29-0 | SDF | Download SDF |
| PubChem ID | 5281055 | Appearance | Powder |
| Formula | C22H23N3O7S | M.Wt | 473.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BRL 49653C | ||
| Solubility | Soluble in DMSO > 10 mM | ||
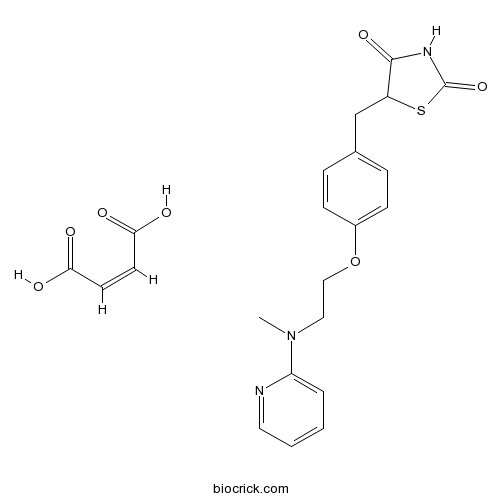
| Chemical Name | (Z)-but-2-enedioic acid;5-[[4-[2-[methyl(pyridin-2-yl)amino]ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione | ||
| SMILES | CN(CCOC1=CC=C(C=C1)CC2C(=O)NC(=O)S2)C3=CC=CC=N3.C(=CC(=O)O)C(=O)O | ||
| Standard InChIKey | SUFUKZSWUHZXAV-BTJKTKAUSA-N | ||
| Standard InChI | InChI=1S/C18H19N3O3S.C4H4O4/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15;5-3(6)1-2-4(7)8/h2-9,15H,10-12H2,1H3,(H,20,22,23);1-2H,(H,5,6)(H,7,8)/b;2-1- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Rosiglitazone maleate is a potent and selective activator of PPARγ, with EC50s of 30 nM, 100 nM and 60 nM for PPARγ1, PPARγ2, and PPARγ, respectively, and a Kd of appr 40 nM for PPARγ; Rosiglitazone maleate is also an modulator of TRP channels, inhibits TRP melastatin 2 (TRPM2), TRPM3 and activates TRP canonical 5 (TRPC5).In Vitro:Rosiglitazone maleate is a potent and selective activator of PPARγ, with EC50s of 30 nM and 100 nM for PPARγ1 and PPARγ2, respectively, and a Kd of appr 40 nM for PPARγ. Rosiglitazone (BRL49653, 0.1, 1,10 μM) promotes differentiation of C3H10T1/2 stem cells to adipocytes[1]. Rosiglitazone (Compound 6) activates PPARγ, with an EC50 of 60 nM[2]. Rosiglitazone (1 μM) activates PPARγ, which binds to NF-α1 promoter to activate gene transcription in neurons. Rosiglitazone (1 μM) also protects Neuro2A cells and hippocampal neurons against oxidative stress, and up-regulates BCL-2 expression in an NF-α1-dependent manner[3]. Rosiglitazone completely inhibits TRPM3 with IC50 values of 9.5 and 4.6 μM against nifedipine- and PregS-evoked activity, but such effects are not via PPARγ. Rosiglitazone inhibits TRPM2 at higher concentration, with an IC50 of appr 22.5 μM. Rosiglitazone is a strong stimulator of TRPC5 channels, with an EC50 of ∼30 μM[4].In Vivo:Rosiglitazone (5 mg/kg, p.o.) decreases the serum glucose in diabetic rats. Rosiglitazone also decreases IL-6, TNF-α, and VCAM-1 levels in diabetic group. Rosiglitazone in combination with losartan increases glucose compared to diabetic and Los-treated groups. Rosiglitazone significantly ameliorates endothelial dysfunction indicated by a significantly lower contractile response to PE and Ang II and enhancement of ACh-provoked relaxation in aortas isolated from diabetic rats[5]. References: | |||||

Rosiglitazone maleate Dilution Calculator

Rosiglitazone maleate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1119 mL | 10.5597 mL | 21.1193 mL | 42.2386 mL | 52.7983 mL |
| 5 mM | 0.4224 mL | 2.1119 mL | 4.2239 mL | 8.4477 mL | 10.5597 mL |
| 10 mM | 0.2112 mL | 1.056 mL | 2.1119 mL | 4.2239 mL | 5.2798 mL |
| 50 mM | 0.0422 mL | 0.2112 mL | 0.4224 mL | 0.8448 mL | 1.056 mL |
| 100 mM | 0.0211 mL | 0.1056 mL | 0.2112 mL | 0.4224 mL | 0.528 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Rosiglitazone maleate is the maleic acid salt form of rosiglitazone, a potent insulin sensitizer, which is used for the treatment of diabetes. Rosiglitazone belongs to a group of compounds, thiazolidinediones (TZDs), which selectively ligate the nuclear transcription factor peroxisome-proliferator-activated receptor-γ (PPARγ). As a TZD, rosiglitazone improves the sensitivity of end organs to insulin through PPARγ activation which poteniates them to lower concentrations of blood glucose. Despite of its beneficial effects of targeting insulin resistance, recent studies has shown that rosiglitazone is associated with several moderate to severe adverse effects, including hemodilution, anemia, weight gain, and edema as well as increased risk for heart failure and myocardial ischemic events.
Reference
Andreas Pfutzner, Birgit Wilhelm, and Thomas Forst. Rosiglitazone and glimeperide: review of clinical results supporting a fixed dose conbination. Vascular Health and Risk Management 2007: 3(2) 211-220
Adie Vilioen and Alan Sinclair. Safety and efficacy of rosiglitazone in the elderly diabetic patient. Vascular Health and Risk Management 2009:5 389-395
- 2-[1-(4-Piperonyl)piperazinyl]benzothiazole
Catalog No.:BCC6771
CAS No.:155106-73-3
- 6-ethyl-3-methyl-4-oxo-4H-pyran-2-carboxylic acid
Catalog No.:BCC8270
CAS No.:1551-49-1
- 11-Deoxyalisol B
Catalog No.:BCN3359
CAS No.:155073-73-7
- 24,25-Dihydroxycycloartan-3-one
Catalog No.:BCN1692
CAS No.:155060-48-3
- SM-21 maleate
Catalog No.:BCC6780
CAS No.:155059-42-0
- N-[2-(Piperidinylamino)ethyl]-4-iodobenzamide
Catalog No.:BCC6784
CAS No.:155054-42-5
- 4-Hydroxy-3-(3-methyl-2-butenoyl)-5-(3-methyl-2-butenyl)benzoic acid
Catalog No.:BCN1554
CAS No.:155051-85-7
- Usaramine
Catalog No.:BCN2121
CAS No.:15503-87-4
- Isatidine
Catalog No.:BCN2120
CAS No.:15503-86-3
- Pancuronium dibromide
Catalog No.:BCC4578
CAS No.:15500-66-0
- Ac-Arg-OH.2H2O
Catalog No.:BCC2855
CAS No.:155-84-0
- Rhaponiticin
Catalog No.:BCN5392
CAS No.:155-58-8
- Plerixafor 8HCl (AMD3100 8HCl)
Catalog No.:BCC4447
CAS No.:155148-31-5
- Plerixafor octahydrobromide
Catalog No.:BCC9123
CAS No.:155148-32-6
- Physapruin A
Catalog No.:BCN7576
CAS No.:155178-03-3
- Cordifolioside A
Catalog No.:BCN8224
CAS No.:155179-20-7
- Cinnamylideneacetic acid
Catalog No.:BCN7777
CAS No.:1552-94-9
- 4-(Dimethylamino)cinnamic acid
Catalog No.:BCN5031
CAS No.:1552-96-1
- 7alpha,15-Dihydroxydehydroabietic acid
Catalog No.:BCN7672
CAS No.:155205-64-4
- Methyl 7,15-dihydroxydehydroabietate
Catalog No.:BCN1693
CAS No.:155205-65-5
- Bimatoprost
Catalog No.:BCC4948
CAS No.:155206-00-1
- Ritonavir
Catalog No.:BCC3620
CAS No.:155213-67-5
- Peraksine
Catalog No.:BCN1694
CAS No.:15527-80-7
- Istradefylline (KW-6002)
Catalog No.:BCC3798
CAS No.:155270-99-8
Biodegradable anionic acrylic resin based hollow microspheres of moderately water soluble drug rosiglitazone maleate: preparation and in vitro characterization.[Pubmed:22356275]
Drug Dev Ind Pharm. 2012 Dec;38(12):1460-9.
BACKGROUND: Most of floating systems have an inbuilt limitation of high variability in the gastric retention time, invariably affecting the bioavailability of drug. An oral sustained release system is formulated to increase gastric residence time by a different way like floating. AIM: The objective of present investigation was to prepare hollow microspheres of Rosiglitazone maleate in order to increase its bioavailability and reduce the dose frequency. METHOD: Hollow microspheres of Rosiglitazone maleate were prepared by O/W emulsion-solvent diffusion technique using biodegradable anionic acrylic resin as a polymer. A mixture of dichloromethane and ethanol (1:1) used as solvent system for drug and polymer with water containing polyvinyl alcohol and salt as external aqueous phase. RESULT: Entrapment efficiency of drug was increased upto 89.71% as a result of salting out effect. The morphology of Eudragit S100 based microspheres in comparison to Ethyl cellulose and hydroxy propyl methyl cellulose (HPMC) was found to be hollow, spherical, and porous which was analysed by scanning electron microscopy. Microspheres were evaluated for micromeritic profile and found satisfactory. The FT-IR spectra confirmed the absence of drug-polymer interaction. The Eudragit S100 based formulation demonstrated favorable in vitro floating and sustained release profile for longer period of time with increased bioavailability. The anionic acrylic resin based microspheres confirmed to have high floating ability >12 h. CONCLUSION: Entrapment efficiency and bioavailability of Rosiglitazone maleate loaded microspheres were increased significantly after modification of method. The release mechanism for formulation was diffusion controlled and had followed first order kinetics, as well as physically and chemically stable as per ICH guidelines.
Preparation and characterization of superporous hydrogels as gastroretentive drug delivery system for rosiglitazone maleate.[Pubmed:22615618]
Daru. 2010;18(3):200-10.
BACKGROUND AND THE PURPOSE OF THE STUDY: Many drugs which have narrow therapeutic window and are absorbed mainly in stomach have been developed as gastroretentive delivery system. Rosiglitazone maleate, an anti-diabetic, is highly unstable at basic pH and is extensively absorbed from the stomach. Hence there is a need to develop a gastroretentive system. In this study a superporous hydrogel was developed as a gastroretentive drug delivery system. METHODS: Chitosan/poly(vinyl alcohol) interpenetrating polymer network type superporous hydrogels were prepared using a gas foaming method employing glyoxal as the crosslinking agent for Rosiglitazone maleate. Sodium bicarbonate was applied as a foaming agent to introduce the porous structure. Swelling behaviors of superporous hydrogel in acidic solution were studied to investigate their applications for gastric retention device. The optimum preparation condition of superporous hydrogels was obtained from the gelation kinetics. FT-IR, scanning electron microscopy, porosity and swelling ratio studies were used to characterize these polymers. In vitro drug release studies were also carried out. RESULTS: The introduction of a small amount of Poly(Vinyl Alcohol) enhanced the mechanical strength but slightly reduced the swelling ratio. The prepared superporous hydrogels were highly sensitive to pH of swelling media, and showed reversible swelling and de-swelling behaviors maintaining their mechanical stability. The degradation kinetics in simulated gastric fluid showed that it had biodegradability. Swelling was dependent on the amount of chitosan and crosslinker. The drug release from superporous hydrogels was sustained for 6 hrs. MAJOR CONCLUSION: The studies showed that chitosan-based superporous hydrogels could be used as a gastroretentive drug delivery system for Rosiglitazone maleate in view of their swelling and prolonged drug release characteristics in acidic pH.
Development and validation of spectrophotometric and HPTLC methods for simultaneous determination of rosiglitazone maleate and metformin hydrochloride in the presence of interfering matrix excipients.[Pubmed:23829222]
Drug Dev Ind Pharm. 2014 Sep;40(9):1190-8.
Two simple methods have been developed and validated for the simultaneous determination of Rosiglitazone maleate (ROS) and metformin hydrochloride (MET) in synthetic mixtures and coated tablets in a ratio of 1:250 (ROS:MET). The first method was a spectrophotometric one. The minor component, ROS was determined by measuring the values of absorbance at lambdamax 312 nm and the D1 amplitudes at 331 nm where MET shows no absorption contribution. However, absorbance interferences from tablet excipients were successfully corrected by D1 at 331 nm zero-crossing technique. Study of spectral interference from tablet excipients was included in the text. Standard curves for Amax and D1 methods were in the concentration range 20.0-80.0 mug mL(-1). The major component, MET was determined both in binary mixtures and tablets by measuring its Amax at 236 nm. Extensive dilution eliminated any absorption contribution from the coexisting ROS or tablet matrix. Standard curves showed linearity in the concentration range 4.0-12.8 mug mL(-1). The second method was based on high performance thin layer chromatography (HPTLC) separation of the two drugs followed by densitometric measurements of their spots at 230 nm. The separation was carried out on Merck HPTLC aluminium sheets of silica gel 60 F254 using methanol:water:NH4Cl 1% w/v (5:4:1 v/v/v) as the mobile phase. Linear calibration graphs of peak area values were obtained versus concentrations in the range of 0.4-2.0 mug band(-1) and 20.0-100.0 mug band(-1) for ROS and MET, respectively. According to International Conference on Harmonisation (ICH) guidelines, different validation parameters were verified for the two methods and presented.
Preparation of rosiglitazone maleate sustained-release floating microspheres for improved bioavailability.[Pubmed:20662314]
Pharmazie. 2010 Jul;65(7):477-80.
The object of this study was to prepare Rosiglitazone maleate (RM) sustained-release floating microspheres and investigate their pharmacokinetics. RM microspheres were prepared with ethyl cellulose (EC) and octadecyl alcohol as the carrier materials by an emulsion-solvent diffusion method, and the properties of morphology in vitro floating capability, drug loading (DL), entrapment efficiency (EE), in vitro release and in vivo pharmacokinetics were investigated. The prepared microspheres had a completely spherical shape. The percentage of microspheres floating after 12 h was (91.45 +/- 1.62)%, and the DL and EE were (9.31 +/- 0.31)% and (89.55 +/- 1.65)% respectively. Pharmacokinetic studies demonstrated that the RM floating microspheres were superior to commercial tablets in terms of the decrease in peak plasma concentration and maintenance of RM concentration in plasma. The area under the curve of plasma concentration-time (AUC) of the floating microspheres was equivalent to that of reference tablets. The results showed that floating microspheres are a feasible approach for the sustained-release preparation of drugs which have limited absorption sites in the upper small intestine.


