Methyl 7,15-dihydroxydehydroabietateCAS# 155205-65-5 |
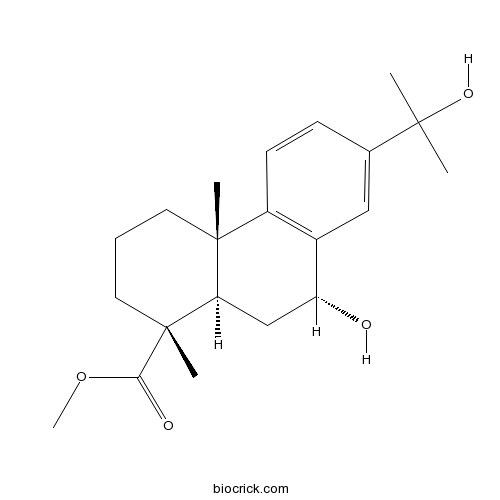
- Methyl 7beta,15-dihydroxydehydroabietate
Catalog No.:BCN7270
CAS No.:107752-10-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 155205-65-5 | SDF | Download SDF |
| PubChem ID | 91895316 | Appearance | Powder |
| Formula | C21H30O4 | M.Wt | 346.5 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | methyl (1R,4aS,9R,10aR)-9-hydroxy-7-(2-hydroxypropan-2-yl)-1,4a-dimethyl-2,3,4,9,10,10a-hexahydrophenanthrene-1-carboxylate | ||
| SMILES | CC12CCCC(C1CC(C3=C2C=CC(=C3)C(C)(C)O)O)(C)C(=O)OC | ||
| Standard InChIKey | MRTXWRPVFGYIEY-DEPWHIHDSA-N | ||
| Standard InChI | InChI=1S/C21H30O4/c1-19(2,24)13-7-8-15-14(11-13)16(22)12-17-20(15,3)9-6-10-21(17,4)18(23)25-5/h7-8,11,16-17,22,24H,6,9-10,12H2,1-5H3/t16-,17-,20-,21-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Methyl 7,15-dihydroxydehydroabietate Dilution Calculator

Methyl 7,15-dihydroxydehydroabietate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.886 mL | 14.43 mL | 28.86 mL | 57.7201 mL | 72.1501 mL |
| 5 mM | 0.5772 mL | 2.886 mL | 5.772 mL | 11.544 mL | 14.43 mL |
| 10 mM | 0.2886 mL | 1.443 mL | 2.886 mL | 5.772 mL | 7.215 mL |
| 50 mM | 0.0577 mL | 0.2886 mL | 0.5772 mL | 1.1544 mL | 1.443 mL |
| 100 mM | 0.0289 mL | 0.1443 mL | 0.2886 mL | 0.5772 mL | 0.7215 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 7alpha,15-Dihydroxydehydroabietic acid
Catalog No.:BCN7672
CAS No.:155205-64-4
- 4-(Dimethylamino)cinnamic acid
Catalog No.:BCN5031
CAS No.:1552-96-1
- Cinnamylideneacetic acid
Catalog No.:BCN7777
CAS No.:1552-94-9
- Cordifolioside A
Catalog No.:BCN8224
CAS No.:155179-20-7
- Physapruin A
Catalog No.:BCN7576
CAS No.:155178-03-3
- Plerixafor octahydrobromide
Catalog No.:BCC9123
CAS No.:155148-32-6
- Plerixafor 8HCl (AMD3100 8HCl)
Catalog No.:BCC4447
CAS No.:155148-31-5
- Rosiglitazone maleate
Catalog No.:BCC2262
CAS No.:155141-29-0
- 2-[1-(4-Piperonyl)piperazinyl]benzothiazole
Catalog No.:BCC6771
CAS No.:155106-73-3
- 6-ethyl-3-methyl-4-oxo-4H-pyran-2-carboxylic acid
Catalog No.:BCC8270
CAS No.:1551-49-1
- 11-Deoxyalisol B
Catalog No.:BCN3359
CAS No.:155073-73-7
- 24,25-Dihydroxycycloartan-3-one
Catalog No.:BCN1692
CAS No.:155060-48-3
- Bimatoprost
Catalog No.:BCC4948
CAS No.:155206-00-1
- Ritonavir
Catalog No.:BCC3620
CAS No.:155213-67-5
- Peraksine
Catalog No.:BCN1694
CAS No.:15527-80-7
- Istradefylline (KW-6002)
Catalog No.:BCC3798
CAS No.:155270-99-8
- NB-598 Maleate
Catalog No.:BCC1788
CAS No.:155294-62-5
- Alisol E 23-acetate
Catalog No.:BCN3459
CAS No.:155301-58-9
- (S)-Sulforaphane
Catalog No.:BCC8097
CAS No.:155320-20-0
- Fluconazole hydrate
Catalog No.:BCC4235
CAS No.:155347-36-7
- p-Menthane-1,3,8-triol
Catalog No.:BCN1695
CAS No.:155348-06-4
- Btk inhibitor 1 R enantiomer hydrochloride
Catalog No.:BCC5126
CAS No.:1553977-42-6
- Ethyl (E)-3'-hydroxy-4'-methoxycinnamate
Catalog No.:BCN3302
CAS No.:155401-23-3
- 4-[2-(2-Amino-4,7-dihydro-4-oxo-1H-pymol[2,3-d]pyrimodin-5-yl)ethyl]benzoic acid methyl ester
Catalog No.:BCC8671
CAS No.:155405-80-4
Feedback regulation of methyl methanesulfonate and ultraviolet-sensitive gene clone 81 via ATM/Chk2 pathway contributes to the resistance of MCF-7 breast cancer cells to cisplatin.[Pubmed:28347251]
Tumour Biol. 2017 Mar;39(3):1010428317694307.
The methyl methanesulfonate and ultraviolet-sensitive gene clone 81 protein is a structure-specific nuclease that plays important roles in DNA replication and repair. Knockdown of methyl methanesulfonate and ultraviolet-sensitive gene clone 81 has been found to sensitize cancer cells to chemotherapy. However, the underlying molecular mechanism is not well understood. We found that methyl methanesulfonate and ultraviolet-sensitive gene clone 81 was upregulated and the ATM/Chk2 pathway was activated at the same time when MCF-7 cells were treated with cisplatin. By using lentivirus targeting methyl methanesulfonate and ultraviolet-sensitive gene clone 81 gene, we showed that knockdown of methyl methanesulfonate and ultraviolet-sensitive gene clone 81 enhanced cell apoptosis and inhibited cell proliferation in MCF-7 cells under cisplatin treatment. Abrogation of ATM/Chk2 pathway inhibited cell viability in MCF-7 cells in response to cisplatin. Importantly, we revealed that ATM/Chk2 was required for the upregulation of methyl methanesulfonate and ultraviolet-sensitive gene clone 81, and knockdown of methyl methanesulfonate and ultraviolet-sensitive gene clone 81 resulted in inactivation of ATM/Chk2 pathway in response to cisplatin. Meanwhile, knockdown of methyl methanesulfonate and ultraviolet-sensitive gene clone 81 activated the p53/Bcl-2 pathway in response to cisplatin. These data suggest that the ATM/Chk2 may promote the repair of DNA damage caused by cisplatin by sustaining methyl methanesulfonate and ultraviolet-sensitive gene clone 81, and the double-strand breaks generated by methyl methanesulfonate and ultraviolet-sensitive gene clone 81 may activate the ATM/Chk2 pathway in turn, which provide a novel mechanism of how methyl methanesulfonate and ultraviolet-sensitive gene clone 81 modulates DNA damage response and repair.
Identification of the Clinical Candidate (R)-(1-(4-Fluorophenyl)-6-((1-methyl-1H-pyrazol-4-yl)sulfonyl)-4,4a,5,6,7,8-hexah ydro-1H-pyrazolo[3,4-g]isoquinolin-4a-yl)(4-(trifluoromethyl)pyridin-2-yl)methano ne (CORT125134): A Selective Glucocorticoid Receptor (GR) Antagonist.[Pubmed:28368581]
J Med Chem. 2017 Apr 27;60(8):3405-3421.
The nonselective glucocorticoid receptor (GR) antagonist mifepristone has been approved in the U.S. for the treatment of selected patients with Cushing's syndrome. While this drug is highly effective, lack of selectivity for GR leads to unwanted side effects in some patients. Optimization of the previously described fused azadecalin series of selective GR antagonists led to the identification of CORT125134, which is currently being evaluated in a phase 2 clinical study in patients with Cushing's syndrome.
Design, synthesis and potent cytotoxic activity of novel 7-(N-[(substituted-sulfonyl)piperazinyl]-methyl)-camptothecin derivatives.[Pubmed:28285912]
Bioorg Med Chem Lett. 2017 Apr 15;27(8):1750-1753.
In an effort to discover potent camptothecin-derived antitumor agents, novel camptothecin analogues with sulfonylpiperazinyl motifs at position-7 were designed and synthesized. They were evaluated for in vitro cytotoxicity with the sulforhodamine-B (SRB) method in five types of human tumor cell lines, A-549, MDA-MB-231, KB, KB-VIN and MCF-7. With IC50 values in the low muM to nM level, most of the new analogues showed greater cytotoxicity activity than the reference compounds irinotecan and topotecan. Furthermore, compounds 12l (IC50, 1.2nM) and 12k (IC50, 20.2nM) displayed the highest cytotoxicity against the multidrug-resistant (MDR) KB-VIN cell line and merit further development as preclinical drug candidates for treating cancer, including MDR phenotype. Our study suggested that integration of sulfonylpiperazinyl motifs into position-7 of camptothecin is an effective strategy for discovering new potent cytotoxic camptothecin derivatives.


