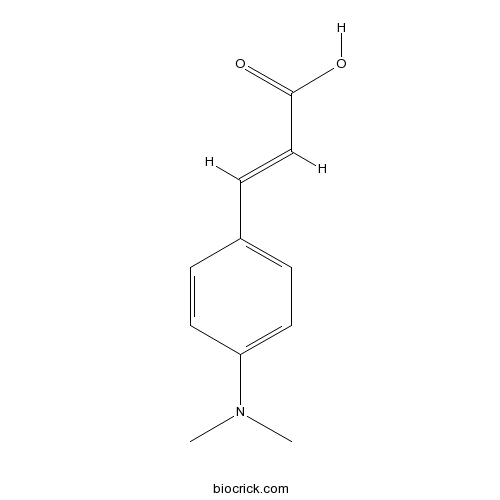
4-(Dimethylamino)cinnamic acidCAS# 1552-96-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1552-96-1 | SDF | Download SDF |
| PubChem ID | 1540638 | Appearance | White cryst. |
| Formula | C11H13NO2 | M.Wt | 191.23 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E)-3-[4-(dimethylamino)phenyl]prop-2-enoic acid | ||
| SMILES | CN(C)C1=CC=C(C=C1)C=CC(=O)O | ||
| Standard InChIKey | CQNPVMCASGWEHM-VMPITWQZSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 4-(Dimethylamino)cinnamic acid (DMACA) could be as charge transfer (ICT) probe. |
| In vitro | Interaction of cinnamic acid derivatives with serum albumins: a fluorescence spectroscopic study.[Pubmed: 21247795]Spectrochim Acta A Mol Biomol Spectrosc. 2011 Mar;78(3):942-8.Cinnamic acid (CA) derivatives are known to possess broad therapeutic applications including anti-tumor activity. The present study was designed to determine the underlying mechanism and thermodynamic parameters for the binding of two CA based intramolecular charge transfer (ICT) fluorescent probes, namely, 4-(dimethylamino) cinnamic acid (DMACA) and trans-ethyl p-(dimethylamino) cinnamate (EDAC), with albumins by fluorescence spectroscopy. |
| Structure Identification | Photochem Photobiol Sci. 2008 Sep;7(9):1063-70.Fluorimetric studies on the binding of 4-(dimethylamino)cinnamic acid with micelles and bovine serum albumin.[Pubmed: 18754053]The constrained photophysics of intramolecular charge transfer (ICT) probe 4-(Dimethylamino)cinnamic acid (DMACA) was studied in different surfactant systems as well as in presence of model water soluble protein bovine serum albumin (BSA). |

4-(Dimethylamino)cinnamic acid Dilution Calculator

4-(Dimethylamino)cinnamic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.2293 mL | 26.1465 mL | 52.2931 mL | 104.5861 mL | 130.7326 mL |
| 5 mM | 1.0459 mL | 5.2293 mL | 10.4586 mL | 20.9172 mL | 26.1465 mL |
| 10 mM | 0.5229 mL | 2.6147 mL | 5.2293 mL | 10.4586 mL | 13.0733 mL |
| 50 mM | 0.1046 mL | 0.5229 mL | 1.0459 mL | 2.0917 mL | 2.6147 mL |
| 100 mM | 0.0523 mL | 0.2615 mL | 0.5229 mL | 1.0459 mL | 1.3073 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cinnamylideneacetic acid
Catalog No.:BCN7777
CAS No.:1552-94-9
- Cordifolioside A
Catalog No.:BCN8224
CAS No.:155179-20-7
- Physapruin A
Catalog No.:BCN7576
CAS No.:155178-03-3
- Plerixafor octahydrobromide
Catalog No.:BCC9123
CAS No.:155148-32-6
- Plerixafor 8HCl (AMD3100 8HCl)
Catalog No.:BCC4447
CAS No.:155148-31-5
- Rosiglitazone maleate
Catalog No.:BCC2262
CAS No.:155141-29-0
- 2-[1-(4-Piperonyl)piperazinyl]benzothiazole
Catalog No.:BCC6771
CAS No.:155106-73-3
- 6-ethyl-3-methyl-4-oxo-4H-pyran-2-carboxylic acid
Catalog No.:BCC8270
CAS No.:1551-49-1
- 11-Deoxyalisol B
Catalog No.:BCN3359
CAS No.:155073-73-7
- 24,25-Dihydroxycycloartan-3-one
Catalog No.:BCN1692
CAS No.:155060-48-3
- SM-21 maleate
Catalog No.:BCC6780
CAS No.:155059-42-0
- N-[2-(Piperidinylamino)ethyl]-4-iodobenzamide
Catalog No.:BCC6784
CAS No.:155054-42-5
- 7alpha,15-Dihydroxydehydroabietic acid
Catalog No.:BCN7672
CAS No.:155205-64-4
- Methyl 7,15-dihydroxydehydroabietate
Catalog No.:BCN1693
CAS No.:155205-65-5
- Bimatoprost
Catalog No.:BCC4948
CAS No.:155206-00-1
- Ritonavir
Catalog No.:BCC3620
CAS No.:155213-67-5
- Peraksine
Catalog No.:BCN1694
CAS No.:15527-80-7
- Istradefylline (KW-6002)
Catalog No.:BCC3798
CAS No.:155270-99-8
- NB-598 Maleate
Catalog No.:BCC1788
CAS No.:155294-62-5
- Alisol E 23-acetate
Catalog No.:BCN3459
CAS No.:155301-58-9
- (S)-Sulforaphane
Catalog No.:BCC8097
CAS No.:155320-20-0
- Fluconazole hydrate
Catalog No.:BCC4235
CAS No.:155347-36-7
- p-Menthane-1,3,8-triol
Catalog No.:BCN1695
CAS No.:155348-06-4
- Btk inhibitor 1 R enantiomer hydrochloride
Catalog No.:BCC5126
CAS No.:1553977-42-6
Interaction of cinnamic acid derivatives with serum albumins: a fluorescence spectroscopic study.[Pubmed:21247795]
Spectrochim Acta A Mol Biomol Spectrosc. 2011 Mar;78(3):942-8.
Cinnamic acid (CA) derivatives are known to possess broad therapeutic applications including anti-tumor activity. The present study was designed to determine the underlying mechanism and thermodynamic parameters for the binding of two CA based intramolecular charge transfer (ICT) fluorescent probes, namely, 4-(dimethylamino) cinnamic acid (DMACA) and trans-ethyl p-(dimethylamino) cinnamate (EDAC), with albumins by fluorescence spectroscopy. Stern-Volmer analysis of the tryptophan fluorescence quenching data in presence of the added ligand reveals fluorescence quenching constant (kappa(q)), Stern-Volmer constant (K(SV)) and also the ligand-protein association constant (K(a)). The thermodynamic parameters like enthalpy (DeltaH) and entropy (DeltaS) change corresponding to the ligand binding process were also estimated. The results show that the ligands bind into the sub-domain IIA of the proteins in 1:1 stoichiometry with an apparent binding constant value in the range of 10(4) dm(3) mol(-1). In both the cases, the spontaneous ligand binding to the proteins occur through entropy driven mechanism, although the interaction of DMACA is relatively stronger in comparison with EDAC. The temperature dependence of the binding constant indicates the induced change in protein secondary structure.
Fluorimetric studies on the binding of 4-(dimethylamino)cinnamic acid with micelles and bovine serum albumin.[Pubmed:18754053]
Photochem Photobiol Sci. 2008 Sep;7(9):1063-70.
The constrained photophysics of intramolecular charge transfer (ICT) probe 4-(Dimethylamino)cinnamic acid (DMACA) was studied in different surfactant systems as well as in presence of model water soluble protein bovine serum albumin (BSA). Binding of the probe in ionic micelles like sodium dodecyl sulfate (SDS) and cetyl trimethyl ammonium bromide (CTAB) causes an increase in ICT fluorescence intensity, whereas, in non-ionic TritonX-100 (TX-100) the intensity decreases with a concomitant increase in emission from locally excited (LE) state. The observations were explained in terms of the different binding affinity, location of the probe and also the nature of specific hydrogen bonding interaction in the excited state nonradiative relaxation process of DMACA. The ICT fluorescence emission yield decreases in BSA due to the locking in of the probe buried in the hydrophobic pocket of the protein structure. SDS induced uncoiling of protein and massive cooperative binding between BSA and SDS is manifested by the release of probe molecules in relatively free aqueous environment.


