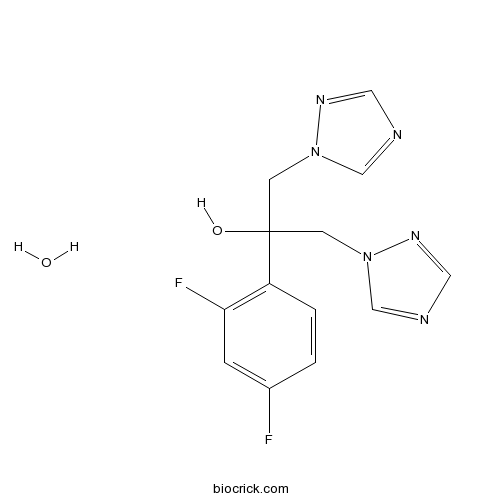
Fluconazole hydrateCAS# 155347-36-7 |
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 155347-36-7 | SDF | Download SDF |
| PubChem ID | 9862025 | Appearance | Powder |
| Formula | C13H14F2N6O2 | M.Wt | 324.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >16.4mg/mL in DMSO | ||
| Chemical Name | 2-(2,4-difluorophenyl)-1,3-bis(1,2,4-triazol-1-yl)propan-2-ol;hydrate | ||
| SMILES | C1=CC(=C(C=C1F)F)C(CN2C=NC=N2)(CN3C=NC=N3)O.O | ||
| Standard InChIKey | QSZFKRINVAUJGQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H12F2N6O.H2O/c14-10-1-2-11(12(15)3-10)13(22,4-20-8-16-6-18-20)5-21-9-17-7-19-21;/h1-3,6-9,22H,4-5H2;1H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Fluconazole hydrate Dilution Calculator

Fluconazole hydrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0837 mL | 15.4183 mL | 30.8366 mL | 61.6732 mL | 77.0915 mL |
| 5 mM | 0.6167 mL | 3.0837 mL | 6.1673 mL | 12.3346 mL | 15.4183 mL |
| 10 mM | 0.3084 mL | 1.5418 mL | 3.0837 mL | 6.1673 mL | 7.7091 mL |
| 50 mM | 0.0617 mL | 0.3084 mL | 0.6167 mL | 1.2335 mL | 1.5418 mL |
| 100 mM | 0.0308 mL | 0.1542 mL | 0.3084 mL | 0.6167 mL | 0.7709 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Fluconazole (hydrate) is a triazole antifungal drug used in the treatment and prevention of superficial and systemic fungal infections.
- (S)-Sulforaphane
Catalog No.:BCC8097
CAS No.:155320-20-0
- Alisol E 23-acetate
Catalog No.:BCN3459
CAS No.:155301-58-9
- NB-598 Maleate
Catalog No.:BCC1788
CAS No.:155294-62-5
- Istradefylline (KW-6002)
Catalog No.:BCC3798
CAS No.:155270-99-8
- Peraksine
Catalog No.:BCN1694
CAS No.:15527-80-7
- Ritonavir
Catalog No.:BCC3620
CAS No.:155213-67-5
- Bimatoprost
Catalog No.:BCC4948
CAS No.:155206-00-1
- Methyl 7,15-dihydroxydehydroabietate
Catalog No.:BCN1693
CAS No.:155205-65-5
- 7alpha,15-Dihydroxydehydroabietic acid
Catalog No.:BCN7672
CAS No.:155205-64-4
- 4-(Dimethylamino)cinnamic acid
Catalog No.:BCN5031
CAS No.:1552-96-1
- Cinnamylideneacetic acid
Catalog No.:BCN7777
CAS No.:1552-94-9
- Cordifolioside A
Catalog No.:BCN8224
CAS No.:155179-20-7
- p-Menthane-1,3,8-triol
Catalog No.:BCN1695
CAS No.:155348-06-4
- Btk inhibitor 1 R enantiomer hydrochloride
Catalog No.:BCC5126
CAS No.:1553977-42-6
- Ethyl (E)-3'-hydroxy-4'-methoxycinnamate
Catalog No.:BCN3302
CAS No.:155401-23-3
- 4-[2-(2-Amino-4,7-dihydro-4-oxo-1H-pymol[2,3-d]pyrimodin-5-yl)ethyl]benzoic acid methyl ester
Catalog No.:BCC8671
CAS No.:155405-80-4
- 4,5-Dihydroblumenol A
Catalog No.:BCN1696
CAS No.:155418-97-6
- JWH 015
Catalog No.:BCC5744
CAS No.:155471-08-2
- 3,6-Caryolanediol
Catalog No.:BCN1697
CAS No.:155485-76-0
- 1,9-Caryolanediol 9-acetate
Catalog No.:BCN1698
CAS No.:155488-34-9
- DIPPA hydrochloride
Catalog No.:BCC6799
CAS No.:155512-52-0
- Alisol F
Catalog No.:BCN3360
CAS No.:155521-45-2
- Alisol G
Catalog No.:BCN3461
CAS No.:155521-46-3
- 3a-Epiburchellin
Catalog No.:BCN7015
CAS No.:155551-61-4
Efficient synthesis of novel 3-aryl-5-(4-chloro-2-morpholinothiazol-5-yl)-4,5-dihydro-1H-pyrazoles and their antifungal activity alone and in combination with commercial antifungal agents.[Pubmed:24895219]
Arch Pharm (Weinheim). 2014 Aug;347(8):566-75.
The alpha,beta-unsaturated carbonyl compounds 5a-f were prepared by reaction between 2-chloro-4-morpholinothiazol-5-carbaldehyde 3 and substituted acetophenones 4a-f. Treatment of compounds 5a-f with hydrazine hydrate employing mild reaction conditions led to the formation of 4,5-dihydro-1H-pyrazoles 6a-f. Then the treatment with acetic anhydride or formic acid afforded the expected 4,5-dihydro-1H-pyrazoles 7a-f and 8a-f. The antifungal activity of each series of synthesized compounds was determined against the clinically important fungi Candida albicans and Cryptococcus neoformans. In addition, the most active compounds 7e and 7f were tested in combination with the commercial antifungal agents: fluconazole, itraconazole, and amphotericin B. Compound 7e showed a synergistic effect with fluconazole against C. albicans while 7f showed synergistic activities with all tested antifungal drugs against the same yeast.
Synthesis of benzofuran based 1,3,5-substituted pyrazole derivatives: as a new class of potent antioxidants and antimicrobials--a novel accost to amend biocompatibility.[Pubmed:22695127]
Bioorg Med Chem Lett. 2012 Jul 15;22(14):4773-7.
In search for a new antioxidant and antimicrobial agent with improved potency, we synthesized a series of benzofuran based 1,3,5-substituted pyrazole analogues (5a-l) in five step reaction. Initially, o-alkyl derivative of salicyaldehyde readily furnish corresponding 2-acetyl benzofuran 2 in good yield, on treatment with 1,8-diaza bicyclo[5.4.0]undec-7-ene (DBU) in the presence of molecular sieves. Further, aldol condensation with vanillin, Claisen-Schmidt condensation reaction with hydrazine hydrate followed by coupling of substituted anilines afforded target compounds. The structures of newly synthesized compounds were confirmed by IR, (1)H NMR, (13)C NMR, mass, elemental analysis and further screened for their antioxidant and antimicrobial activities. Among the tested compounds 5d and 5f exhibited good antioxidant property with 50% inhibitory concentration higher than that of reference while compounds 5h and 5l exhibited good antimicrobial activity at concentration 1.0 and 0.5 mg/mL compared with standard, streptomycin and fluconazole respectively.
Facile heterocyclic synthesis and antimicrobial activity of polysubstituted and condensed pyrazolopyranopyrimidine and pyrazolopyranotriazine derivatives.[Pubmed:26677897]
Acta Pharm. 2015 Dec;65(4):399-412.
Reaction of 6-amino-3-methyl-4-(substituted phenyl)-1,4- dihydropyrano[2,3-c]pyrazole-5-carbonitrile (1) with triethylorthoformate followed by treatment with hydrazine hydrate, formic acid, acetic acid, phenylisocyanate, ammonium thiocyanate and formamide afforded the corresponding pyranopyrimidine derivatives 2-6. Cyclocondensation of 1 with cyclohexanone afforded pyrazolopyranoquinoline 7. One-pot process of diazotation and de-diazochlorination of 1 afforded pyrazolopyranotriazine derivative 8, which upon treatment with secondary amines afforded 9 and 10a- c. Condensation of 2 with aromatic aldehyde gave the corresponding Schiff bases 11a,b, the oxidative cyclization of the hydrazone with appropriate oxidant afforded 11-(4- fluorophenyl))- 2-(4-substituted phenyl)-10-methyl-8,11-dihydropyrazolo-[ 4',3':5,6]pyrano[3,2-e][1,2,4]triazolo[1,5-c]pyrimidines (12a,b). Structures of the synthesized compounds were confirmed by spectral data and elemental analysis. All synthesized compounds were evaluated for antibacterial and antifungal activities compared to norfloxacin and fluconazole as standard drugs. Compounds 9, 10c, 12a and 15 were found to be the most potent antibacterial agents, with activity equal to that of norfloxacin. On the other hand, compound 5 exhibited higher antifungal activity compared to fluconazole.
Exclusive plaque psoriasis of the lips: efficacy of combination therapy of topical tacrolimus, calcipotriol, and betamethasone dipropionate.[Pubmed:22779103]
Skinmed. 2012 May-Jun;10(3):183-4.
A 16-year-old unmarried woman presented with recurrent cracking of the lips indicated by the appearance of grayish white flakes since October 2004, which, in due course, shed off leaving behind an apparently normal mucous membrane. Chewing roasted corn treated with salt and lemon (bhutta) initially caused the lesions. Ever since, it has been a cause of its exacerbation. She never had any relief with either systemic or topical treatment. In fact, an obsession had overtaken her, resulting in a psychological setback. She denied regular drug use for any other ailment. Her menstrual cycle was normal. There was a positive history of psoriasis in her mother. Examination of the lips was conspicuous. It was marked by the presence of a well-circumscribed, moist, raised plaque (Figure 1). Its surface was irregular, with elevation and depression. It was made up of thick, grayish white scales, which were arranged in layers; however, Grattage/Auspitz sign could not be elicited. Fissuring was prominent but the buccal mucosa, surface of the tongue, gingiva, and palate were normal. The clinical examination did not reveal any evidence of skin and/or nail psoriasis/psoriatic arthropathy or any other systemic abnormality. Blood examination including total and differential leukocyte count, complete hemogram, and liver and renal function tests were normal. Biopsy of the representative lesion was subjected to serial sections. They were stained with hematoxylin-eosin to work up microscopic pathology. It revealed the presence of mounds of parakeratosis with numerous neutrophilic Munro microabscesses (Figure 2). Submucosal vessels were dilated and congested. Periodic-acid-Schiff (PAS) stain revealed fungal hyphae and spores within the parakeratotic layer. Colonies of Gram-positive cocci were also demonstrated on the surface of the mucosa. She was administered combination therapy, comprising topical tacrolimus (0.1%) ointment and calcipotirol hydrate (50 microg/g) plus betmethasone dipropionate (0.5 mg/g) twice a day for 7 days. A single bolus dose of fluconazole 450 mg orally was also administered. The response to treatment was favorable and the lesions showed regression (Figure 3).
Synthesis and antimicrobial activity of some novel oxadiazole derivatives.[Pubmed:19051585]
Acta Pol Pharm. 2008 Jul-Aug;65(4):441-7.
A series of 5-(3'-oxo-6'-(substituted aryl)-2',3',4',5'-tetrahydropyridazin-2'-ylmethyl )-2-substituted 1,3,4-oxadiazole has been synthesized. Appropriate aromatic hydrocarbon reacts with succinic anhydride in the presence of AICl3 to yield beta-aroyl propionic acid (1a). The corresponding acid is cyclized with hydrazine hydrate to give 6-(substituted aryl)-2,3,4,5-tetrahydro-3-pyridazinone (1b). This ntermediate after reaction with ethyl bromoacetate, was hydrazinolyzed into 3-oxo-6-(substituted aryl)-2, 3, 4, 5-tetrahydropyridazinyl acetohydrazide (1c). The resulting product was converted into 5-[3'-oxo-6'-(substituted aryl)-2',3',4',5'-tetrahydropyridazin-2'-ylmethyl]-2-substituted 1,3,4-oxadiazole (Scheme 1). All the final compounds were structurally elucidated on the basis of IR, H-NMR, MS data and elemental analysis and screened for antibacterial, antifungal and antitubercular activity. All the compounds are evaluated for their antibacterial activity against E. coli, S. aureus, Micrococcus luteus and Klebsiella pneumoniae by using cup plate technique in the nutrient agar at 100 microg/mL concentration. Antitubercular activity was determined using the BACTEC 460 system. Stock solutions of test compounds were prepared in DMSO. MIC of rifampin was calculated by established procedures. All the synthesized compounds were screened at 6.25 microg/mL against M. tuberculosis H37 Rv comparable with that of standard rifampicin and isoniazid. All the final compounds were evaluated for antifungal activity against C. albicans and C. neoformans by using cup-plate method in the Sabouraud agar media The zone of inhibition (mm) of each compound was determined and compared with standard drug fluconazole.


