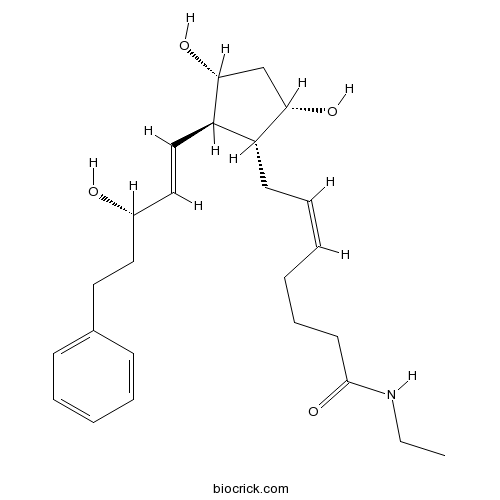
BimatoprostCAS# 155206-00-1 |
- CHIR-99021 (CT99021)
Catalog No.:BCC1275
CAS No.:252917-06-9
- SB 415286
Catalog No.:BCC3651
CAS No.:264218-23-7
- SB 216763
Catalog No.:BCC3650
CAS No.:280744-09-4
- AR-A014418
Catalog No.:BCC1366
CAS No.:487021-52-3
- LY2090314
Catalog No.:BCC1717
CAS No.:603288-22-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 155206-00-1 | SDF | Download SDF |
| PubChem ID | 5311027 | Appearance | Powder |
| Formula | C25H37NO4 | M.Wt | 415.57 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AGN 192024 | ||
| Solubility | DMSO : ≥ 100 mg/mL (240.63 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(E,3S)-3-hydroxy-5-phenylpent-1-enyl]cyclopentyl]-N-ethylhept-5-enamide | ||
| SMILES | CCNC(=O)CCCC=CCC1C(CC(C1C=CC(CCC2=CC=CC=C2)O)O)O | ||
| Standard InChIKey | AQOKCDNYWBIDND-FTOWTWDKSA-N | ||
| Standard InChI | InChI=1S/C25H37NO4/c1-2-26-25(30)13-9-4-3-8-12-21-22(24(29)18-23(21)28)17-16-20(27)15-14-19-10-6-5-7-11-19/h3,5-8,10-11,16-17,20-24,27-29H,2,4,9,12-15,18H2,1H3,(H,26,30)/b8-3-,17-16+/t20-,21+,22+,23-,24+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Bimatoprost is a prostaglandin analog used topically (as eye drops) to control the progression of glaucoma and in the management of ocular hypertension.
Target: Prostaglandin Receptor
Bimatoprost is a prostaglandin analog/prodrug used topically (as eye drops) to control the progression of glaucoma and in the management of ocular hypertension. It reduces intraocular pressure (IOP) by increasing the outflow of aqueous fluid from the eyes. In December 2008, the indication to lengthen eyelashes was approved by the U.S. Food and Drug Administration (FDA) the cosmetic formulation of bimatoprost is sold as Latisse. In 2008-2011, at least three case series suggested that bimatoprost has the ability to reduce adipose (fat) tissue.
Bimatoprost activates prostamide alpha F2 receptors found in the hair follicle to stimulate its growth rate. Research led by Professor Randall and the University of Bradford found that it may also offer a treatment for scalp hair regrowth in trials conducted on samples taken from men undergoing hair transplants. According to Allergan's package labeling, users of its Latisse cosmetic product didn't develop darker irises in clinical studies however, patients should be advised about the potential for increased brown iris pigmentation which is likely to be permanent. References: | |||||

Bimatoprost Dilution Calculator

Bimatoprost Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4063 mL | 12.0317 mL | 24.0633 mL | 48.1267 mL | 60.1583 mL |
| 5 mM | 0.4813 mL | 2.4063 mL | 4.8127 mL | 9.6253 mL | 12.0317 mL |
| 10 mM | 0.2406 mL | 1.2032 mL | 2.4063 mL | 4.8127 mL | 6.0158 mL |
| 50 mM | 0.0481 mL | 0.2406 mL | 0.4813 mL | 0.9625 mL | 1.2032 mL |
| 100 mM | 0.0241 mL | 0.1203 mL | 0.2406 mL | 0.4813 mL | 0.6016 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Bimatoprost is a prostaglandin analog used topically (as eye drops) to control the progression of glaucoma and in the management of ocular hypertension.
- Methyl 7,15-dihydroxydehydroabietate
Catalog No.:BCN1693
CAS No.:155205-65-5
- 7alpha,15-Dihydroxydehydroabietic acid
Catalog No.:BCN7672
CAS No.:155205-64-4
- 4-(Dimethylamino)cinnamic acid
Catalog No.:BCN5031
CAS No.:1552-96-1
- Cinnamylideneacetic acid
Catalog No.:BCN7777
CAS No.:1552-94-9
- Cordifolioside A
Catalog No.:BCN8224
CAS No.:155179-20-7
- Physapruin A
Catalog No.:BCN7576
CAS No.:155178-03-3
- Plerixafor octahydrobromide
Catalog No.:BCC9123
CAS No.:155148-32-6
- Plerixafor 8HCl (AMD3100 8HCl)
Catalog No.:BCC4447
CAS No.:155148-31-5
- Rosiglitazone maleate
Catalog No.:BCC2262
CAS No.:155141-29-0
- 2-[1-(4-Piperonyl)piperazinyl]benzothiazole
Catalog No.:BCC6771
CAS No.:155106-73-3
- 6-ethyl-3-methyl-4-oxo-4H-pyran-2-carboxylic acid
Catalog No.:BCC8270
CAS No.:1551-49-1
- 11-Deoxyalisol B
Catalog No.:BCN3359
CAS No.:155073-73-7
- Ritonavir
Catalog No.:BCC3620
CAS No.:155213-67-5
- Peraksine
Catalog No.:BCN1694
CAS No.:15527-80-7
- Istradefylline (KW-6002)
Catalog No.:BCC3798
CAS No.:155270-99-8
- NB-598 Maleate
Catalog No.:BCC1788
CAS No.:155294-62-5
- Alisol E 23-acetate
Catalog No.:BCN3459
CAS No.:155301-58-9
- (S)-Sulforaphane
Catalog No.:BCC8097
CAS No.:155320-20-0
- Fluconazole hydrate
Catalog No.:BCC4235
CAS No.:155347-36-7
- p-Menthane-1,3,8-triol
Catalog No.:BCN1695
CAS No.:155348-06-4
- Btk inhibitor 1 R enantiomer hydrochloride
Catalog No.:BCC5126
CAS No.:1553977-42-6
- Ethyl (E)-3'-hydroxy-4'-methoxycinnamate
Catalog No.:BCN3302
CAS No.:155401-23-3
- 4-[2-(2-Amino-4,7-dihydro-4-oxo-1H-pymol[2,3-d]pyrimodin-5-yl)ethyl]benzoic acid methyl ester
Catalog No.:BCC8671
CAS No.:155405-80-4
- 4,5-Dihydroblumenol A
Catalog No.:BCN1696
CAS No.:155418-97-6
Bimatoprost-induced late-onset choroidal detachment after trabeculectomy: A case report and review of the literature.[Pubmed:28151873]
Medicine (Baltimore). 2017 Feb;96(5):e5927.
BACKGROUND: Choroidal detachment (CD) is often observed at an early period particularly after trabeculectomy and glaucoma drainage implant surgery. However, topical antiglaucoma eye drop-induced CD is a rare complication. Here, we report a case of topical Bimatoprost-induced late-onset CD after trabeculectomy and review the literature. CASE REPORT: A 74-year-old man who suffered from primary open-angle glaucoma underwent his initial trabeculectomy with mitomycin-C in the right eye. Before the surgery, his intraocular pressure (IOP) was 20 to 22 mm Hg with Bimatoprost 0.03%, dorzolamide 1%, and brimonidine 0.1% and his best corrected visual acuity (BCVA) was 0.9. The mean deviation in Humphrey Visual Field Analyzer (24-2 program) was -27.83 db. After successful trabeculectomy, IOPs were 11 to 16 mm Hg without any medication. Eight months after the surgery, we restarted Bimatoprost to further reduce the IOP in the right eye, which was 15 mm Hg. At a hospital visit 2 months later, he complained of blurred vision that had persisted for the past 1 month; his IOP had decreased to 9 mm Hg. His BCVA was 0.04 and 3 quadrant CD was found. We discontinued Bimatoprost and started him on betamethasone 0.1% 4 times per day. However, CD marginally changed after 1 week, with IOP at 7 mm Hg; thus, we performed scleral drainage for CD. After 3 weeks of drainage, CD completely disappeared. IOP increased to 16 mm Hg and BCVA was 0.7. However, 3 months after the drainage, IOP increased to 29 mm Hg, and needling revision was thus performed. After the surgery, IOP remained at 14 to 16 mm Hg without any glaucoma medication and CD recurrence. A review of the literature showed that various antiglaucoma medications induce CD, regardless of the preceding glaucoma surgery and that CD is usually resolved by withdrawing the medication and administering topical steroids. However, most previous studies have shown the recurrence of CD by rechallenging the same drug. CONCLUSION: In our case, topical Bimatoprost induced late-onset CD after trabeculectomy. Early scleral drainage may be a good option to quickly resolve drug-induced CD and prevent its recurrence. Therefore, it must be kept in mind that various antiglaucoma medications induce CD.
Effects of LATISSE (bimatoprost 0.03 per cent topical solution) on the ocular surface.[Pubmed:28122407]
Clin Exp Optom. 2017 Nov;100(6):583-589.
PURPOSE: LATISSE is marketed for the treatment of hypotrichosis (loss of eyelashes), using a prostamide analogue and preserved with benzalkonium chloride, which is an effective preservative; however, it also causes irritation to the ocular surface. LATISSE is applied to the lid margin; however, with the blink, some solution may fall onto the ocular surface. The objective of this study was to assess the effects of LATISSE on the ocular surface over two months. METHODS: Non-dry eye participants interested in eyelash lengthening were invited to a prospective uncontrolled, open-label clinical study using LATISSE for two months. Eyelash length, subjective symptoms, tear film stability, osmolarity, ocular redness and intraocular pressure were evaluated at baseline (T0) and at one (T1) and two months (T2). RESULTS: Twenty-eight women (ages 18 to 29) entered the study. Fifteen completed the study with five who discontinued due to burning upon instillation and eight were lost to follow-up. Average eyelash length increased at each time (p < 0.001). Dryness, burning and grittiness remained low (less than 25/100) throughout the trial with dryness showing a significant change between T0 and T1 (p = 0.04), but not between T1 and T2 (p > 0.05). No difference (p > 0.05) was noted for the non-invasive break-up time, photochromametry or tear osmolarity. Intraocular pressure showed a decrease with time but translated to only a one to two mmHg change, which was not clinically relevant. CONCLUSIONS: LATISSE increases eyelash length within a short time (less than two months). Patients seeking eyelash enhancement options should be educated as to the use, precautions and any secondary effects, including the potential for discomfort upon instillation.
In Vivo Effects of Retrobulbar Bimatoprost Injection on Orbital Fat.[Pubmed:28369019]
Ophthalmic Plast Reconstr Surg. 2018 May/Jun;34(3):201-204.
PURPOSE: Recent publications have reported the adverse effects of prostaglandin analogues on the periocular tissues. These medications may cause periorbital lipodystrophy, enophthalmos, and deepening of the superior sulcus deformity. While these effects may have adverse consequences for some patients, the atrophy of the periorbital fat may have a useful role in diseases that lead to orbital and periorbital fat hypertrophy such as thyroid eye disease. In this pilot study, the authors investigated the effects of retrobulbar Bimatoprost injection on the intraocular pressure and orbital fat in a rat animal model. METHODS: Three rats were sedated and intraocular pressure was measured. A 0.1 ml aliquot of Bimatoprost was injected into the right orbit of all rats. In the left orbit, 0.1 ml of phosphate-buffered saline was injected as a control. Three weeks later, all rats were sedated and intraocular pressure was measured before euthanizing. Routine histologic staining was performed and thin sections through the intraconal orbital fat were obtained. Density of intraconal adipocytes was measured and adipocyte heterogeneity was determined using a computer image analysis algorithm. RESULTS: The specimens injected with Bimatoprost demonstrated atrophy of orbital fat with significantly increased adipocyte density (p = 0.009) and heterogeneity (p = 0.008) when compared with control. Intraocular pressure was not significantly decreased at 3 weeks after injection of retrobulbar Bimatoprost. CONCLUSIONS: In this pilot study, orbital injection of Bimatoprost demonstrated atrophy of intraconal adipocytes when compared with control orbits injected with saline. The orbits injected with Bimatoprost were noted to have smaller, more heterogeneous adipocytes that were densely packed in the intraconal space. The study limitations include the small sample size, which limited the ability for us to make conclusions about the effect on intraocular pressure. Nevertheless, the findings presented suggest that retrobulbar Bimatoprost may present a nonsurgical alternative to induce atrophy of the orbital fat without inducing inflammation or hypotony.
Bimatoprost for the treatment of eyelash, eyebrow and scalp alopecia.[Pubmed:28264599]
Expert Opin Investig Drugs. 2017 Apr;26(4):515-522.
INTRODUCTION: Alopecia is a common condition observed among people of all ages. It is a disorder that can involve only the scalp as observed in androgenetic alopecia or scalp and body as in alopecia areata or patients under chemotherapy treatment. There are several treatment options with different safety and efficacy outcomes. Bimatoprost, a synthetic prostamide F2alpha analog originally approved for the treatment of ocular hypertension and open-angle glaucoma, is now FDA approved as a 0.03%, solution to be applied once daily to increase eyelashes growth. Areas covered: In this review, the authors evaluate the role of Bimatoprost in idiopathic hypotrichosis of the eyelashes, in hypotrichosis of the eyelashes associated to chemotherapy, in alopecia areata of the eyelashes and eyebrows and in androgenetic alopecia. In addition, pharmacokinetics, pharmacodynamics, safety and tolerability of Bimatoprost are discussed. Expert opinion: Bimatoprost will likely be the third FDA approved weapon in the fight against hair loss. Prostaglandin analogs are the only possible treatment for hypotrichosis and alopecia of the eyelashes regardless of its etiology. Eyebrow hypotrichosis due to alopecia areata or frontal fibrosis alopecia can also possibly benefit of these medications.


