PeraksineCAS# 15527-80-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 15527-80-7 | SDF | Download SDF |
| PubChem ID | 78146432 | Appearance | Powder |
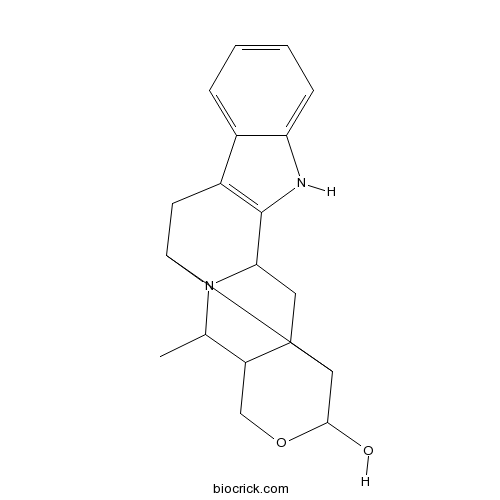
| Formula | C19H22N2O2 | M.Wt | 310.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 21-methyl-18-oxa-1,11-diazahexacyclo[11.8.0.02,16.04,12.05,10.015,20]henicosa-4(12),5,7,9-tetraen-17-ol | ||
| SMILES | CC1C2COC(C3C2CC4N1C3CC5=C4NC6=CC=CC=C56)O | ||
| Standard InChIKey | LVOPRJWLXUCHRL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H22N2O2/c1-9-13-8-23-19(22)17-11(13)6-16-18-12(7-15(17)21(9)16)10-4-2-3-5-14(10)20-18/h2-5,9,11,13,15-17,19-20,22H,6-8H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | J Org Chem. 2014 Nov 7;79(21):10030-48.General strategy for synthesis of C-19 methyl-substituted sarpagine/macroline/ajmaline indole alkaloids including total synthesis of 19(S),20(R)-dihydroperaksine, 19(S),20(R)-dihydroperaksine-17-al, and peraksine.[Pubmed: 25247616]
J Ethnopharmacol. 1984 Jun;11(1):99-117.Stem bark alkaloids of Rauvolfia caffra.[Pubmed: 6471882]
|

Peraksine Dilution Calculator

Peraksine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2216 mL | 16.1082 mL | 32.2165 mL | 64.433 mL | 80.5412 mL |
| 5 mM | 0.6443 mL | 3.2216 mL | 6.4433 mL | 12.8866 mL | 16.1082 mL |
| 10 mM | 0.3222 mL | 1.6108 mL | 3.2216 mL | 6.4433 mL | 8.0541 mL |
| 50 mM | 0.0644 mL | 0.3222 mL | 0.6443 mL | 1.2887 mL | 1.6108 mL |
| 100 mM | 0.0322 mL | 0.1611 mL | 0.3222 mL | 0.6443 mL | 0.8054 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ritonavir
Catalog No.:BCC3620
CAS No.:155213-67-5
- Bimatoprost
Catalog No.:BCC4948
CAS No.:155206-00-1
- Methyl 7,15-dihydroxydehydroabietate
Catalog No.:BCN1693
CAS No.:155205-65-5
- 7alpha,15-Dihydroxydehydroabietic acid
Catalog No.:BCN7672
CAS No.:155205-64-4
- 4-(Dimethylamino)cinnamic acid
Catalog No.:BCN5031
CAS No.:1552-96-1
- Cinnamylideneacetic acid
Catalog No.:BCN7777
CAS No.:1552-94-9
- Cordifolioside A
Catalog No.:BCN8224
CAS No.:155179-20-7
- Physapruin A
Catalog No.:BCN7576
CAS No.:155178-03-3
- Plerixafor octahydrobromide
Catalog No.:BCC9123
CAS No.:155148-32-6
- Plerixafor 8HCl (AMD3100 8HCl)
Catalog No.:BCC4447
CAS No.:155148-31-5
- Rosiglitazone maleate
Catalog No.:BCC2262
CAS No.:155141-29-0
- 2-[1-(4-Piperonyl)piperazinyl]benzothiazole
Catalog No.:BCC6771
CAS No.:155106-73-3
- Istradefylline (KW-6002)
Catalog No.:BCC3798
CAS No.:155270-99-8
- NB-598 Maleate
Catalog No.:BCC1788
CAS No.:155294-62-5
- Alisol E 23-acetate
Catalog No.:BCN3459
CAS No.:155301-58-9
- (S)-Sulforaphane
Catalog No.:BCC8097
CAS No.:155320-20-0
- Fluconazole hydrate
Catalog No.:BCC4235
CAS No.:155347-36-7
- p-Menthane-1,3,8-triol
Catalog No.:BCN1695
CAS No.:155348-06-4
- Btk inhibitor 1 R enantiomer hydrochloride
Catalog No.:BCC5126
CAS No.:1553977-42-6
- Ethyl (E)-3'-hydroxy-4'-methoxycinnamate
Catalog No.:BCN3302
CAS No.:155401-23-3
- 4-[2-(2-Amino-4,7-dihydro-4-oxo-1H-pymol[2,3-d]pyrimodin-5-yl)ethyl]benzoic acid methyl ester
Catalog No.:BCC8671
CAS No.:155405-80-4
- 4,5-Dihydroblumenol A
Catalog No.:BCN1696
CAS No.:155418-97-6
- JWH 015
Catalog No.:BCC5744
CAS No.:155471-08-2
- 3,6-Caryolanediol
Catalog No.:BCN1697
CAS No.:155485-76-0
General strategy for synthesis of C-19 methyl-substituted sarpagine/macroline/ajmaline indole alkaloids including total synthesis of 19(S),20(R)-dihydroperaksine, 19(S),20(R)-dihydroperaksine-17-al, and peraksine.[Pubmed:25247616]
J Org Chem. 2014 Nov 7;79(21):10030-48.
A detailed account of the development of a general strategy for synthesis of the C-19 methyl-substituted alkaloids including total synthesis of 19(S),20(R)-dihydroPeraksine-17-al (1), 19(S),20(R)-dihydroPeraksine (2), and Peraksine (6) is presented. Efforts directed toward the total synthesis of macrosalhine chloride (5) are also reported. Important to success is the sequence of chemical reactions which include a critical haloboration reaction, regioselective hydroboration, and controlled oxidation (to provide sensitive enolizable aldehydes at C-20). In addition, the all-important Pd-catalyzed alpha-vinylation reaction has been extended to a chiral C-19 alkyl-substituted substrate for the first time. Synthesis of the advanced intermediate 64 completes an improved formal total synthesis of talcarpine (26) and provides a starting point for synthesis of macroline-related alkaloids 27-31. Similarly, extension of this synthetic strategy in the ring A oxygenated series should provide easy access to the northern hemisphere 32b of the bisindoles angustricraline, alstocraline, and foliacraline (Figure 4 ).
Stem bark alkaloids of Rauvolfia caffra.[Pubmed:6471882]
J Ethnopharmacol. 1984 Jun;11(1):99-117.
Thirty two alkaloids were isolated from the stem bark of Rauvolfia caffra and 28 were identified. The alkaloids represented corynane (3), strictamine (1), sarpagan (4), akuammicine (2), pleiocarpamine (1), indolenine (1), dihydroindole (6), Peraksine (3), heteroyohimbine (2), hydroxyheteroyohimbine (2), oxindole (1), 2-acyl-indole (1), suaveoline (3) and yohimbine (2) types. The anhydronium base serpentine was detected but not isolated. The principal alkaloids were ajmaline and norajmaline (dihydroindoles), ajmalicinine and ajmalicine (heteroyohimbines), geissoschizol (E-seco indole) and pleiocarpamine and the heteroyohimbine derived alkaloids were predominantly normal configuration compounds. The biosynthetic and ethnopharmacological significance of the alkaloids is discussed.


