1,9-Caryolanediol 9-acetateCAS# 155488-34-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 155488-34-9 | SDF | Download SDF |
| PubChem ID | 91895382 | Appearance | Powder |
| Formula | C17H28O3 | M.Wt | 280.4 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
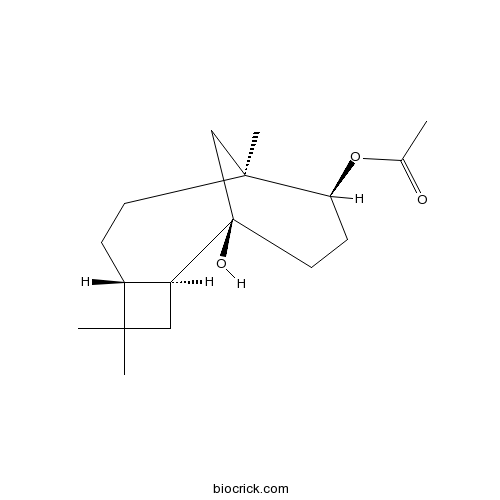
| Chemical Name | [(1R,2S,5R,8S,9S)-1-hydroxy-4,4,8-trimethyl-9-tricyclo[6.3.1.02,5]dodecanyl] acetate | ||
| SMILES | CC(=O)OC1CCC2(CC1(CCC3C2CC3(C)C)C)O | ||
| Standard InChIKey | LRFYCTLMXJJJHZ-UAHISNFZSA-N | ||
| Standard InChI | InChI=1S/C17H28O3/c1-11(18)20-14-6-8-17(19)10-16(14,4)7-5-12-13(17)9-15(12,2)3/h12-14,19H,5-10H2,1-4H3/t12-,13+,14+,16+,17-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

1,9-Caryolanediol 9-acetate Dilution Calculator

1,9-Caryolanediol 9-acetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5663 mL | 17.8317 mL | 35.6633 mL | 71.3267 mL | 89.1583 mL |
| 5 mM | 0.7133 mL | 3.5663 mL | 7.1327 mL | 14.2653 mL | 17.8317 mL |
| 10 mM | 0.3566 mL | 1.7832 mL | 3.5663 mL | 7.1327 mL | 8.9158 mL |
| 50 mM | 0.0713 mL | 0.3566 mL | 0.7133 mL | 1.4265 mL | 1.7832 mL |
| 100 mM | 0.0357 mL | 0.1783 mL | 0.3566 mL | 0.7133 mL | 0.8916 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3,6-Caryolanediol
Catalog No.:BCN1697
CAS No.:155485-76-0
- JWH 015
Catalog No.:BCC5744
CAS No.:155471-08-2
- 4,5-Dihydroblumenol A
Catalog No.:BCN1696
CAS No.:155418-97-6
- 4-[2-(2-Amino-4,7-dihydro-4-oxo-1H-pymol[2,3-d]pyrimodin-5-yl)ethyl]benzoic acid methyl ester
Catalog No.:BCC8671
CAS No.:155405-80-4
- Ethyl (E)-3'-hydroxy-4'-methoxycinnamate
Catalog No.:BCN3302
CAS No.:155401-23-3
- Btk inhibitor 1 R enantiomer hydrochloride
Catalog No.:BCC5126
CAS No.:1553977-42-6
- p-Menthane-1,3,8-triol
Catalog No.:BCN1695
CAS No.:155348-06-4
- Fluconazole hydrate
Catalog No.:BCC4235
CAS No.:155347-36-7
- (S)-Sulforaphane
Catalog No.:BCC8097
CAS No.:155320-20-0
- Alisol E 23-acetate
Catalog No.:BCN3459
CAS No.:155301-58-9
- NB-598 Maleate
Catalog No.:BCC1788
CAS No.:155294-62-5
- Istradefylline (KW-6002)
Catalog No.:BCC3798
CAS No.:155270-99-8
- DIPPA hydrochloride
Catalog No.:BCC6799
CAS No.:155512-52-0
- Alisol F
Catalog No.:BCN3360
CAS No.:155521-45-2
- Alisol G
Catalog No.:BCN3461
CAS No.:155521-46-3
- 3a-Epiburchellin
Catalog No.:BCN7015
CAS No.:155551-61-4
- Hydroxyfasudil hydrochloride
Catalog No.:BCC1636
CAS No.:155558-32-0
- VUF 10166
Catalog No.:BCC5060
CAS No.:155584-74-0
- Dehydroaglaiastatin
Catalog No.:BCN1699
CAS No.:155595-93-0
- PG-9 maleate
Catalog No.:BCC6779
CAS No.:155649-00-6
- Notoginsenoside Ft1
Catalog No.:BCN6434
CAS No.:155683-00-4
- Simonsinol
Catalog No.:BCN1700
CAS No.:155709-40-3
- Isomagnolone
Catalog No.:BCN1701
CAS No.:155709-41-4
- Mecarbinate
Catalog No.:BCC4919
CAS No.:15574-49-9
Chemical Transformations of the Fungal Meroterpenoid Dhilirolide A Reveal Skeletal Degradation and Rearrangement Reactions with Biosynthetic Implications.[Pubmed:28805391]
Org Lett. 2017 Sep 1;19(17):4488-4491.
Treatment of the fungal meroterpenoid dhilirolide A (1) with either sodium azide or perchloric acid results in conversion of the dhilirane carbon skeleton of 1 to the 14,15-dinordhilirane carbon skeleton of the products 5-7, with and without concomitant transfer of an acetyl residue to form a C-9 acetate ester. The discovery of these transformations, which are vinylogous retro-Claisen-type condensations, suggests an efficient biogenetic route to 14,15-dinordhiliranes such as dhilirolide K (3).
Two new lignans from the resin of Bursera microphylla A. gray and their cytotoxic activity.[Pubmed:28920481]
Nat Prod Res. 2017 Sep 18:1-6.
Two new lignans, namely 7-O-podophyllotoxinyl butyrate (1) and dihydroclusin 9-acetate (2), were isolated from the dichloromethane fraction of a methanol extract of Bursera microphylla (Burseraceae), along with eight known lignans (3-10). Their structures were determined by means of comprehensive spectroscopic analysis. Lignans 2-6 were tested for their anti-proliferative activity on the cancer cell lines LS180, A549 and HeLa, and on a non-cancer cell line, ARPE-19. Only compounds 4 and 5 showed an interesting activity on HeLa cells.
[Lignans from cultivated Gynura nepalensis].[Pubmed:28884539]
Zhongguo Zhong Yao Za Zhi. 2016 Apr;41(8):1456-1460.
Taking application of some isolation and purification technologies, including solvent extraction, rude solvent isolation, column chromatographies on silica gel and Sephadex LH-20 , and preparative HPLC , 4 compounds were obtained from Gynura nepalensis cultivated in a suburban area of Beijing. Their structures were identified by spectroscopic methods in conjunction with comparison of the NMR data with literature values as 7S,8R-9'-O-ethyl-dehydrodiconiferyl-9-acetate (1), 9'-O-ethyl-dehydrodiconiferyl alcohol (2), dehydrodiconiferyl-9,9'-diacetate(3), and (+)-medioresinol(4), respectively. 1 is a new 2,3-dihydrobenzofuran-8,3'-neolignane type compound, and 2-4 were isolated from G.nepalensis for the first time. The complete assignment of the 1H- and 13C-NMR spectroscopic data of the four compounds recorded in DMSO-d6 was achieved.
Phenolics from Mikania micrantha and Their Antioxidant Activity.[Pubmed:28698451]
Molecules. 2017 Jul 8;22(7). pii: molecules22071140.
A phytochemical study on the aerial parts of Mikania micrantha led to the isolation of two new phenolic compounds, benzyl 5-O-beta-d-glucopyranosyl-2,5-dihydroxybenzoate (1) and (7S,8R)-threo-dihydroxydehydrodiconiferyl alcohol 9-acetate (2), together with twelve known compounds, benzyl 2-O-beta-d-glucopyranosyl-2,6-dihydroxybenzoate (3), 4-allyl-2,6-dimethoxyphenol glucoside (4), (+)-isolariciresinol (5), icariol A(2) (6), 9,10-dihydroxythymol (7), 8,9,10-trihydroxythymol (8), caffeic acid (9), p-coumaric acid (10), ethyl protocatechuate (11), procatechuic aldehyde (12), 4-hydroxybenzoic acid (13), and hydroquinone (14). Their structures were elucidated on the basis of extensive spectroscopic analysis. Except 8 and 9, all the other compounds were isolated from this plant species for the first time. The antioxidant activity of those isolated compounds were evaluated using three different assays. Compounds 1, 2, 3, 9, 10, 13, and 14 demonstrated significant 2,2'-azinobis-(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) free radical cation scavenging activity ranging from SC50 0.31 to 4.86 microM, which were more potent than l-ascorbic acid (SC50 = 10.48 microM). Compounds 5, 9, 11, and 12 exhibited more potent 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity (SC50 = 16.24-21.67 microM) than l-ascorbic acid (39.48 microM). Moreover, the ferric reducing antioxidant power (FRAP) of compounds 2, 5, 9, and 11 were discovered to be also comparable to or even more potent than l-ascorbic acid.
Solubility of lead and copper in biochar-amended small arms range soils: influence of soil organic carbon and pH.[Pubmed:23869882]
J Agric Food Chem. 2013 Aug 14;61(32):7679-88.
Biochar is often considered a strong heavy metal stabilizing agent. However, biochar in some cases had no effects on, or increased the soluble concentrations of, heavy metals in soil. The objective of this study was to determine the factors causing some biochars to stabilize and others to dissolve heavy metals in soil. Seven small arms range soils with known total organic carbon (TOC), cation exchange capacity, pH, and total Pb and Cu contents were first screened for soluble Pb and Cu concentrations. Over 2 weeks successive equilibrations using weak acid (pH 4.5 sulfuric acid) and acetate buffer (0.1 M at pH 4.9), Alaska soil containing disproportionately high (31.6%) TOC had nearly 100% residual (insoluble) Pb and Cu. This soil was then compared with sandy soils from Maryland containing significantly lower (0.5-2.0%) TOC in the presence of 10 wt % (i) plant biochar activated to increase the surface-bound carboxyl and phosphate ligands (PS450A), (ii) manure biochar enriched with soluble P (BL700), and (iii) unactivated plant biochars produced at 350 degrees C (CH350) and 700 degrees C (CH500) and by flash carbonization (corn). In weak acid, the pH was set by soil and biochar, and the biochars increasingly stabilized Pb with repeated extractions. In pH 4.9 acetate buffer, PS450A and BL700 stabilized Pb, and only PS450A stabilized Cu. Surface ligands of PS450A likely complexed and stabilized Pb and Cu even under acidic pH in the presence of competing acetate ligand. Oppositely, unactivated plant biochars (CH350, CH500, and corn) mobilized Pb and Cu in sandy soils; the putative mechanism is the formation of soluble complexes with biochar-borne dissolved organic carbon. In summary, unactivated plant biochars can inadvertently increase dissolved Pb and Cu concentrations of sandy, low TOC soils when used to stabilize other contaminants.


