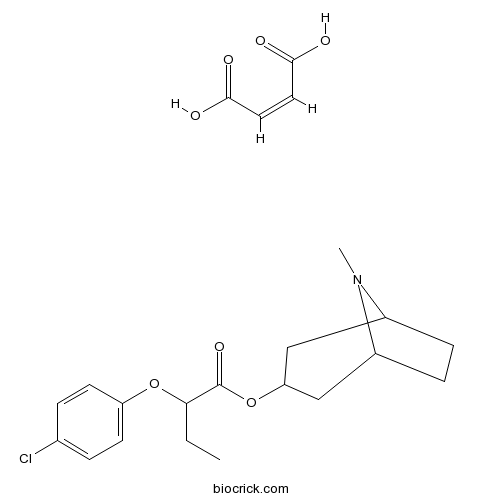
SM-21 maleatePresynaptic cholinergic modulator CAS# 155059-42-0 |
- BV6
Catalog No.:BCC5372
CAS No.:1001600-56-1
- LCL161
Catalog No.:BCC1691
CAS No.:1005342-46-0
- Birinapant (TL32711)
Catalog No.:BCC2250
CAS No.:1260251-31-7
- Embelin
Catalog No.:BCN2678
CAS No.:550-24-3
- GDC-0152
Catalog No.:BCC2252
CAS No.:873652-48-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 155059-42-0 | SDF | Download SDF |
| PubChem ID | 11691005 | Appearance | Powder |
| Formula | C22H28ClNO7 | M.Wt | 453.92 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in water | ||
| Chemical Name | (Z)-but-2-enedioic acid;(8-methyl-8-azabicyclo[3.2.1]octan-3-yl) 2-(4-chlorophenoxy)butanoate | ||
| SMILES | CCC(C(=O)OC1CC2CCC(C1)N2C)OC3=CC=C(C=C3)Cl.C(=CC(=O)O)C(=O)O | ||
| Standard InChIKey | BHXGTFUQDGMXHA-BTJKTKAUSA-N | ||
| Standard InChI | InChI=1S/C18H24ClNO3.C4H4O4/c1-3-17(22-15-8-4-12(19)5-9-15)18(21)23-16-10-13-6-7-14(11-16)20(13)2;5-3(6)1-2-4(7)8/h4-5,8-9,13-14,16-17H,3,6-7,10-11H2,1-2H3;1-2H,(H,5,6)(H,7,8)/b;2-1- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective σ2 antagonist with central effects following systemic administration. Causes increased release of acetylcholine at central muscarinic synapses. Potent analgesic (efficacy comparable to morphine) and nootropic agent. |

SM-21 maleate Dilution Calculator

SM-21 maleate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.203 mL | 11.0152 mL | 22.0303 mL | 44.0606 mL | 55.0758 mL |
| 5 mM | 0.4406 mL | 2.203 mL | 4.4061 mL | 8.8121 mL | 11.0152 mL |
| 10 mM | 0.2203 mL | 1.1015 mL | 2.203 mL | 4.4061 mL | 5.5076 mL |
| 50 mM | 0.0441 mL | 0.2203 mL | 0.4406 mL | 0.8812 mL | 1.1015 mL |
| 100 mM | 0.022 mL | 0.1102 mL | 0.2203 mL | 0.4406 mL | 0.5508 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- N-[2-(Piperidinylamino)ethyl]-4-iodobenzamide
Catalog No.:BCC6784
CAS No.:155054-42-5
- 4-Hydroxy-3-(3-methyl-2-butenoyl)-5-(3-methyl-2-butenyl)benzoic acid
Catalog No.:BCN1554
CAS No.:155051-85-7
- Usaramine
Catalog No.:BCN2121
CAS No.:15503-87-4
- Isatidine
Catalog No.:BCN2120
CAS No.:15503-86-3
- Pancuronium dibromide
Catalog No.:BCC4578
CAS No.:15500-66-0
- Ac-Arg-OH.2H2O
Catalog No.:BCC2855
CAS No.:155-84-0
- Rhaponiticin
Catalog No.:BCN5392
CAS No.:155-58-8
- Methscopolamine
Catalog No.:BCC4577
CAS No.:155-41-9
- RU 58841
Catalog No.:BCC1911
CAS No.:154992-24-2
- Erysenegalensein E
Catalog No.:BCN3979
CAS No.:154992-17-3
- BAY-u 9773
Catalog No.:BCC7576
CAS No.:154978-38-8
- LL 37
Catalog No.:BCC8027
CAS No.:154947-66-7
- 24,25-Dihydroxycycloartan-3-one
Catalog No.:BCN1692
CAS No.:155060-48-3
- 11-Deoxyalisol B
Catalog No.:BCN3359
CAS No.:155073-73-7
- 6-ethyl-3-methyl-4-oxo-4H-pyran-2-carboxylic acid
Catalog No.:BCC8270
CAS No.:1551-49-1
- 2-[1-(4-Piperonyl)piperazinyl]benzothiazole
Catalog No.:BCC6771
CAS No.:155106-73-3
- Rosiglitazone maleate
Catalog No.:BCC2262
CAS No.:155141-29-0
- Plerixafor 8HCl (AMD3100 8HCl)
Catalog No.:BCC4447
CAS No.:155148-31-5
- Plerixafor octahydrobromide
Catalog No.:BCC9123
CAS No.:155148-32-6
- Physapruin A
Catalog No.:BCN7576
CAS No.:155178-03-3
- Cordifolioside A
Catalog No.:BCN8224
CAS No.:155179-20-7
- Cinnamylideneacetic acid
Catalog No.:BCN7777
CAS No.:1552-94-9
- 4-(Dimethylamino)cinnamic acid
Catalog No.:BCN5031
CAS No.:1552-96-1
- 7alpha,15-Dihydroxydehydroabietic acid
Catalog No.:BCN7672
CAS No.:155205-64-4
(+/-)-SM 21 attenuates the convulsive and locomotor stimulatory effects of cocaine in mice.[Pubmed:11301071]
Eur J Pharmacol. 2001 Apr 6;417(1-2):R1-2.
Cocaine interacts with sigma receptors at physiologically relevant concentrations. While earlier studies demonstrate that antagonism of sigma(1) receptors attenuates the behavioral actions of cocaine, the contribution of sigma(2) receptors is unclear. Therefore, in the present study, 3 alpha-tropanyl-2-(4-chlorophenoxy)butyrate ((+/-)-SM 21), a compound with high and preferential affinity for sigma(2) receptors, was tested for its ability to attenuate cocaine-induced behaviors. Pre-treatment of Swiss Webster mice with (+/-)-SM 21 significantly attenuated cocaine-induced convulsions and locomotor activity.
Pharmacological identification of SM-21, the novel sigma(2) antagonist.[Pubmed:11164098]
Pharmacol Biochem Behav. 2000 Nov;67(3):659-62.
SM-21 is a tropane analogue with high affinity and selectivity for sigma(2) receptor subtype. In the absence of highly selective sigma(2) antagonists, the aim of the present study was to determine whether SM-21 is endowed with antagonistic activity. The experiments were conducted in rats by inducing neck dystonia, which is reported to be subsequent to activation of sigma(2) receptors. SM-21 (10 nmol/0.5 microl) was able to prevent torsion of the neck obtained by administration of the sigma(1)-sigma(2) agonist 1,3-di-(2-tolyl)guanidine (DTG, 5 nmol/0.5 microl) in the red nucleus. These data indicate that SM-21 is a potent and selective sigma(2) antagonist.
Antinociceptive profile of 3-alpha-tropanyl 2-(4-Cl-phenoxy)butyrate (SM-21) [corrected]: a novel analgesic with a presynaptic cholinergic mechanism of action.[Pubmed:9223584]
J Pharmacol Exp Ther. 1997 Jul;282(1):430-9.
The antinociceptive effect of (+/-)-3-alpha-tropanyl 2-(4-Cl-phenoxy)butyrate [corrected] (SM-21) (10-40 mg kg(-1) s.c., 10-30 mg kg(-1) i.p., 20-60 mg kg(-1) p.o., 3-20 mg kg(-1) i.v. and 5-20 microg per mouse i.c.v.) was examined in rodents and guinea pigs by using the hot-plate, abdominal constriction, tail-flick and paw-pressure tests. The antinociception produced by (+/-)-SM-21 was prevented by atropine, pirenzepine and hemicholinium-3 but not by quinpirole, R-(alpha)-methylhistamine, [1-[2(methylsufonyl)amino]ethyl]-4-piperidinyl]methyl-5-floro++ +-2-methoxy-1H-indole-3-carboxylate hydrochloride, N6-cyclopentyladenosine, 1-(2-methoxyphenyl)-4-[4-(2-phthalimido)butyl]piperazine hydrobromide, naloxone, 3-aminopropyl-diethoxy-methyl-phosphinic acid or reserpine. On the basis of the above data, it can be postulated that (+/-)-SM-21 exerted an antinociceptive effect mediated by a central potentiation of cholinergic transmission. Affinity profiles of (+/-)-SM-21 for muscarinic receptor subtypes, determined by functional studies (rabbit vas deferens for M1, guinea pig atrium for M2, guinea pig ileum for M3 and immature guinea pig uterus for putative M4) have shown a selectivity ratio M2/M1 of 4.6 that, although very low, might be responsible for the antinociception induced by (+/-)-SM-21 through an increase in ACh extracellular levels. In the antinociceptive dose range, (+/-)-SM-21 did not impair mouse performance evaluated by the rota-rod and hole-board tests.


