BAY-u 9773Antagonist of CysLT1/CysLT2 receptors CAS# 154978-38-8 |
- Vinblastine Sulfate
Catalog No.:BCN2292
CAS No.:143-67-9
- Pepstatin A
Catalog No.:BCC1218
CAS No.:26305-03-3
- Dexamethasone (DHAP)
Catalog No.:BCC1184
CAS No.:50-02-2
- Omeprazole
Catalog No.:BCC1254
CAS No.:73590-58-6
- E 64d
Catalog No.:BCC1127
CAS No.:88321-09-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 154978-38-8 | SDF | Download SDF |
| PubChem ID | 5311015 | Appearance | Powder |
| Formula | C27H36O5S | M.Wt | 472.64 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in ethanol (supplied pre-dissolved in Ethanol, 0.1mg/ml) | ||
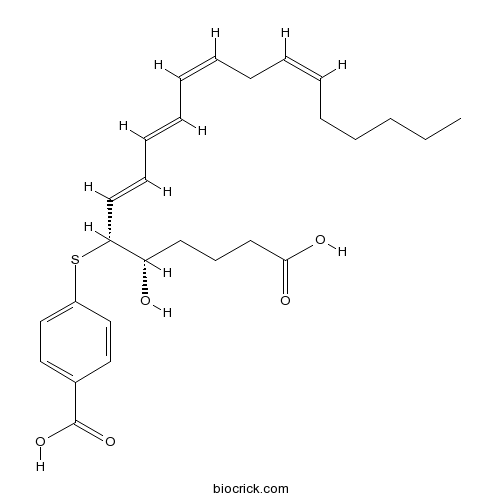
| Chemical Name | 4-[(4S,5R,6E,8E,10Z,13Z)-1-carboxy-4-hydroxynonadeca-6,8,10,13-tetraen-5-yl]sulfanylbenzoic acid | ||
| SMILES | CCCCCC=CCC=CC=CC=CC(C(CCCC(=O)O)O)SC1=CC=C(C=C1)C(=O)O | ||
| Standard InChIKey | PKJINWOACFYDQN-RBVMPENBSA-N | ||
| Standard InChI | InChI=1S/C27H36O5S/c1-2-3-4-5-6-7-8-9-10-11-12-13-16-25(24(28)15-14-17-26(29)30)33-23-20-18-22(19-21-23)27(31)32/h6-7,9-13,16,18-21,24-25,28H,2-5,8,14-15,17H2,1H3,(H,29,30)(H,31,32)/b7-6-,10-9-,12-11+,16-13+/t24-,25+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cysteinyl leukotriene receptor antagonist with equal affinity at both CysLT1 and CysLT2 receptors (pKB values are 6.8 and 6.5 respectively). Competitive antagonist of LTC4- and LTE4-induced contractions of the guinea pig trachea. |

BAY-u 9773 Dilution Calculator

BAY-u 9773 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1158 mL | 10.5789 mL | 21.1578 mL | 42.3155 mL | 52.8944 mL |
| 5 mM | 0.4232 mL | 2.1158 mL | 4.2316 mL | 8.4631 mL | 10.5789 mL |
| 10 mM | 0.2116 mL | 1.0579 mL | 2.1158 mL | 4.2316 mL | 5.2894 mL |
| 50 mM | 0.0423 mL | 0.2116 mL | 0.4232 mL | 0.8463 mL | 1.0579 mL |
| 100 mM | 0.0212 mL | 0.1058 mL | 0.2116 mL | 0.4232 mL | 0.5289 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BAY-u9773, an antagonist of cysteinyl leukotriene (Cys-LT) receptor, which have equal affinity towards CysLT1 and CysLT2 receptors.
The Cys-LTs are a family of potent bioactive lipids, acting through two distinct G protein-coupled receptors named the CysLT1 and CysLT2 receptors.
To determine the profile of BAY-u9773 as a Cys-LT receptor antagonist, the effect of this component on different smooth muscle preparations was investigated. The result showed that BAY u9773 antagonised 'typical' cysteinyl-leukotriene receptors and inhibited bronchial and venous muscle contractions in the human muscle preparations [1].
BAY-u9773 was also extensively used in animal model to study the function of CysLT1 and CysLT2 receptors. For instance, BAY-u9773 treatment inhibited the infiltration of eosinophil in BAL Fluid in a model of OVA-induced airway hypersensitivity and inflammation in guinea pigs [2]. In addition, this components was shown to act as competitive antagonist towards LTC4 and LTE4 receptors and results in contractions in trachea of guinea pig models [3].
References:
1. Tudhope SR, Cuthbert NJ, Abram TS, Jennings MA, Maxey RJ, Thompson AM, et al. BAY u9773, a novel antagonist of cysteinyl-leukotrienes with activity against two receptor subtypes. Eur J Pharmacol 1994,264:317-323.
2. Muraki M, Imbe S, Santo H, Sato R, Sano H, Iwanaga T, et al. Effects of a cysteinyl leukotriene dual 1/2 receptor antagonist on antigen-induced airway hypersensitivity and airway inflammation in a guinea pig asthma model. Int Arch Allergy Immunol 2011,155 Suppl 1:90-95.
3. Wikstrom Jonsson E, Rosenqvist U, Dahlen SE. Agonist and antagonist activities of the leukotriene analogue BAY u9773 in guinea pig lung parenchyma. Eur J Pharmacol 1998,357:203-211.
- LL 37
Catalog No.:BCC8027
CAS No.:154947-66-7
- Kaempferol 3,7,4'-trimethylether
Catalog No.:BCN4087
CAS No.:15486-34-7
- 3,5-Dihydroxy-4',7-dimethoxyflavone
Catalog No.:BCN1691
CAS No.:15486-33-6
- Eleutheroside C
Catalog No.:BCN1690
CAS No.:15486-24-5
- 4-Acetoxycinnamic acid
Catalog No.:BCN5026
CAS No.:15486-19-8
- Cimiside E
Catalog No.:BCN7951
CAS No.:154822-57-8
- 2-(2'-Hydroxytetracosanoylamino)-octadecane-1,3,4-triol
Catalog No.:BCN1555
CAS No.:154801-30-6
- 3-Methoxymollugin
Catalog No.:BCN7164
CAS No.:154706-44-2
- Tezampanel
Catalog No.:BCC1993
CAS No.:154652-83-2
- Efavirenz
Catalog No.:BCC4135
CAS No.:154598-52-4
- Stauprimide
Catalog No.:BCC7768
CAS No.:154589-96-5
- SB 204990
Catalog No.:BCC6342
CAS No.:154566-12-8
- Erysenegalensein E
Catalog No.:BCN3979
CAS No.:154992-17-3
- RU 58841
Catalog No.:BCC1911
CAS No.:154992-24-2
- Methscopolamine
Catalog No.:BCC4577
CAS No.:155-41-9
- Rhaponiticin
Catalog No.:BCN5392
CAS No.:155-58-8
- Ac-Arg-OH.2H2O
Catalog No.:BCC2855
CAS No.:155-84-0
- Pancuronium dibromide
Catalog No.:BCC4578
CAS No.:15500-66-0
- Isatidine
Catalog No.:BCN2120
CAS No.:15503-86-3
- Usaramine
Catalog No.:BCN2121
CAS No.:15503-87-4
- 4-Hydroxy-3-(3-methyl-2-butenoyl)-5-(3-methyl-2-butenyl)benzoic acid
Catalog No.:BCN1554
CAS No.:155051-85-7
- N-[2-(Piperidinylamino)ethyl]-4-iodobenzamide
Catalog No.:BCC6784
CAS No.:155054-42-5
- SM-21 maleate
Catalog No.:BCC6780
CAS No.:155059-42-0
- 24,25-Dihydroxycycloartan-3-one
Catalog No.:BCN1692
CAS No.:155060-48-3
Diets and trophic-guild structure of a diverse fish assemblage in Chesapeake Bay, U.S.A.[Pubmed:25627041]
J Fish Biol. 2015 Mar;86(3):967-92.
Dietary habits and trophic-guild structure were examined in a fish assemblage (47 species) of the Chesapeake Bay estuary, U.S.A., using 10 years of data from >25 000 fish stomachs. The assemblage was comprised of 10 statistically significant trophic guilds that were principally differentiated by the relative amounts of Mysida, Bivalvia, Polychaeta, Teleostei and other Crustacea in the diets. These guilds were broadly aggregated into five trophic categories: piscivores, zooplanktivores, benthivores, crustacivores and miscellaneous consumers. Food web structure was largely dictated by gradients in habitat (benthic to pelagic) and prey size. Size classes within piscivorous species were more likely to be classified into different guilds, reflecting stronger dietary changes through ontogeny relative to benthivores and other guilds. Relative to predator species and predator size, the month of sampling had negligible effects on dietary differences within the assemblage. A majority of sampled fishes derived most of their nutrition from non-pelagic prey sources, suggesting a strong coupling of fish production to benthic and demersal food resources. Mysida (predominantly the opossum shrimp Neomysis americana) contributed substantially to the diets of over 25% of the sampled predator groups, indicating that this species is a critical, but underappreciated, node in the Chesapeake Bay food web.
Correction: Evaluating the Operational Features of an Unconventional Dual-Bay U-Turn Design for Intersections.[Pubmed:27657538]
PLoS One. 2016 Sep 22;11(9):e0163758.
[This corrects the article DOI: 10.1371/journal.pone.0158914.].
Evaluating the Operational Features of an Unconventional Dual-Bay U-Turn Design for Intersections.[Pubmed:27467127]
PLoS One. 2016 Jul 28;11(7):e0158914.
Median U-turn intersection treatment (MUTIT) has been considered an alternative measure to reduce congestion and traffic conflict at intersection areas. The MUTIT is sometimes difficult to implement in the field because it requires wide median on arterials for U-turn vehicles. The objective of this study is to introduce an unconventional U-turn treatment (UUT) for intersections which requires less median space but is also effective. The UUT has a dual-bay design with different turning radiuses for small and large vehicles. The VISSIM simulation model was developed to evaluate the operational features of the UUT. The model was calibrated using data collected from intersections in China. The capacity, delay and number of stops were evaluated and compared with the direct-left-turn (DLT) for the same intersections. The results showed that the UUT significantly improved the operations at intersection areas, especially when volume/capacity ratio is small, and ratio of left-turn to through traffic is small. With the UUT, the capacity is increased by 9.81% to 10.38%, vehicle delay is decreased by 18.5% to 40.1%, and number of stops is decreased by 23.19% to 36.62%, when volume/capacity ratio is less than 0.50. The study also found that traffic efficiency could be further improved when the UUT is designed in conjunction with signal control. In the case, the UUT plus signalized control increases the capacity by 25% to 26.02%, decreases vehicle delay by 50.5% to 55.8%, and reduces number of stops by 69.5%, compared with the traditional DLT.
From Sediment to Top Predators: Broad Exposure of Polyhalogenated Carbazoles in San Francisco Bay (U.S.A.).[Pubmed:28112952]
Environ Sci Technol. 2017 Feb 21;51(4):2038-2046.
The present study provides the first comprehensive investigation of polyhalogenated carbazoles (PHCZs) contamination in an aquatic ecosystem. PHCZs have been found in soil and aquatic sediment from several different regions, but knowledge of their bioaccumulation and trophodynamics is extremely scarce. This work investigated a suite of 11 PHCZ congeners in San Francisco Bay (United States) sediment and organisms, including bivalves (n = 6 composites), sport fish (n = 12 composites), harbor seal blubber (n = 18), and bird eggs (n = 8 composites). The most detectable congeners included 3,6-dichlorocarbazole (36-CCZ), 3,6-dibromocarbazole (36-BCZ), 1,3,6-tribromocarbazole (136-BCZ), 1,3,6,8-tetrabromocarbazole (1368-BCZ), and 1,8-dibromo-3,6-dichlorocarbazole (18-B-36-CCZ). The median concentrations of SigmaPHCZs were 9.3 ng/g dry weight in sediment and ranged from 33.7 to 164 ng/g lipid weight in various species. Biomagnification was observed from fish to harbor seal and was mainly driven by chlorinated carbazoles, particularly 36-CCZ. Congener compositions of PHCZs differed among species, suggesting that individual congeners may be subject to different bioaccumulation or metabolism in species occupying various trophic levels in the studied aquatic system. Toxic equivalent (TEQ) values of PHCZs were determined on the basis of their relative effect potencies (REP) compared to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). The median TEQ was 1.2 pg TEQ/g dry weight in sediment and 4.8-19.5 pg TEQ/g lipid weight in biological tissues. Our study demonstrated the broad exposure of PHCZs in San Francisco Bay and their characteristics of bioaccumulation and biomagnification along with dioxin-like effects. These findings raise the need for additional research to better elucidate their sources, environmental behavior, and fate in global environments.
Functional characteristics of cysteinyl-leukotriene receptor subtypes.[Pubmed:12072150]
Life Sci. 2002 Jun 28;71(6):611-22.
Cysteinyl-leukotrienes, i.e. leukotriene (LT) C4, D4 and E4, are inflammatory mediators and potent airway- and vasoconstrictors. Two different cysteinyl-leukotriene receptors, CysLT1 and CysLT2, have been cloned and functionally characterised using potent CysLT1 receptor antagonists and the dual CysLT1/CysLT2 receptor antagonist BAY u9773. However, the rank order of potency of the cysteinyl-leukotrienes at the CysLT receptors differs between tissues and studies, and a CysLT receptor classification based on agonist selectivity has not been established. In addition, the existence of more than two receptor subtypes for cysteinyl-leukotrienes has been suggested.
A second cysteinyl leukotriene receptor in human lung.[Pubmed:1331415]
J Pharmacol Exp Ther. 1992 Nov;263(2):800-5.
Leukotrienes (LT) are potent spasmogenic agents in human isolated bronchial and pulmonary venous muscle preparations. Treatment of human isolated pulmonary veins with the L-serine borate complex (45 mM; 30 min) did not alter the LTC4 pD2 values in these preparations. The cysteinyl LT antagonists, ICI 198615, MK 571 and SKF 104353, significantly shifted to the right the LT concentration-effect curves in airways with pKB values against LTC4 of 8.4 for ICI 198615, 8.6 for MK 571 and 8.0 for SKF 104353. Similar results were found against LTD4. In contrast, these antagonists did not inhibit the LTC4 and LTD4 contractions in human pulmonary veins. LTE4 was a partial agonist on the human pulmonary veins and blocked the contractions with a pKp value of 6.3 against LTD4 and 6.6 against LTC4. An LT analog, BAY u9773, also blocked the LT contractions in bronchial and venous muscle preparations with pKp values against LTD4 and LTC4 of 6.5 and 6.7, respectively. These data provide pharmacological evidence for a second cysteinyl LT receptor in the human lung. One LT receptor (LT-1) is stimulated by all cysteinyl LT, found on airways and inhibited by the LT-1 antagonists, and a second receptor (LT-2) can also be stimulated by all cysteinyl LT and is found on pulmonary veins, resistant to LT-1 antagonists but blocked by LTE4 and the dual LT-1/LT-2 antagonist BAY u9773.


