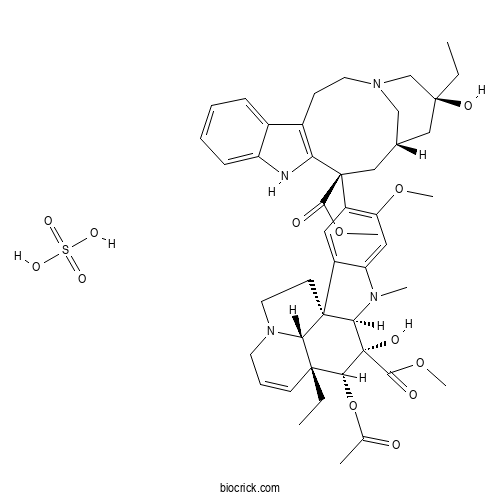
Vinblastine SulfateAnti-mitotic agent CAS# 143-67-9 |
- Doxorubicin
Catalog No.:BCC2082
CAS No.:23214-92-8
- Pepstatin A
Catalog No.:BCC1218
CAS No.:26305-03-3
- Dexamethasone (DHAP)
Catalog No.:BCC1184
CAS No.:50-02-2
- Omeprazole
Catalog No.:BCC1254
CAS No.:73590-58-6
- E 64d
Catalog No.:BCC1127
CAS No.:88321-09-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 143-67-9 | SDF | Download SDF |
| PubChem ID | 241902 | Appearance | Powder |
| Formula | C46H60N4O13S | M.Wt | 909.06 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Vincaleukoblastine sulfate salt | ||
| Solubility | DMSO : ≥ 44 mg/mL (48.40 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | methyl (1R,9R,10S,11R,12R,19R)-11-acetyloxy-12-ethyl-4-[(13S,15S,17S)-17-ethyl-17-hydroxy-13-methoxycarbonyl-1,11-diazatetracyclo[13.3.1.04,12.05,10]nonadeca-4(12),5,7,9-tetraen-13-yl]-10-hydroxy-5-methoxy-8-methyl-8,16-diazapentacyclo[10.6.1.01,9.02,7.016,19]nonadeca-2,4,6,13-tetraene-10-carboxylate;sulfuric acid | ||
| SMILES | CCC1(CC2CC(C3=C(CCN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5)C78CCN9C7C(C=CC9)(C(C(C8N6C)(C(=O)OC)O)OC(=O)C)CC)OC)C(=O)OC)O.OS(=O)(=O)O | ||
| Standard InChIKey | KDQAABAKXDWYSZ-JKDPCDLQSA-N | ||
| Standard InChI | InChI=1S/C46H58N4O9.H2O4S/c1-8-42(54)23-28-24-45(40(52)57-6,36-30(15-19-49(25-28)26-42)29-13-10-11-14-33(29)47-36)32-21-31-34(22-35(32)56-5)48(4)38-44(31)17-20-50-18-12-16-43(9-2,37(44)50)39(59-27(3)51)46(38,55)41(53)58-7;1-5(2,3)4/h10-14,16,21-22,28,37-39,47,54-55H,8-9,15,17-20,23-26H2,1-7H3;(H2,1,2,3,4)/t28-,37+,38-,39-,42+,43-,44-,45+,46+;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Vinblastine Sulfate has anticancer activity, using FA-BSANPs as a drug carrier system could be effective in targeting Vinblastine Sulfate-sensitive tumors in the future. Vinblastine Sulfate could offer protection to the normal tissues against gamma-radiation-induced DNA strand breaks. The changes induced on the rabbit OM by Vinblastine sulphate are transient and that regenerative recovery leads to the restoration of the normal structure of the mucosa. |
| Targets | DNA/RNA Synthesis |
| In vitro | Effect of vinblastine sulfate on gamma-radiation-induced DNA single-strand breaks in murine tissues.[Pubmed: 12694742]Mutat Res. 2003 Apr 20;536(1-2):15-25.The effect of Vinblastine Sulfate on gamma-radiation-induced DNA strand breaks in different tissues of tumour bearing mice, was studied by single-cell gel electrophoresis. |
| In vivo | Anticancer drug vinblastine sulphate induces transient morphological changes on the olfactory mucosa of the rabbit.[Pubmed: 22443492]Anat Histol Embryol. 2012 Oct;41(5):374-87.Vinblastine Sulfate (VBS) is an anticancer drug that acts by disrupting microtubule dynamics of highly mitotic tissue cells. The consequences of Vinblastine sulphate on the olfactory mucosa (OM), a tissue with high mitotic numbers, are not clearly understood. A comparison of nitrogen mustard and vinblastine sulfate in the treatment of patients with Hodgkin's disease.[Pubmed: 5414540]Can Med Assoc J. 1970 Feb 14;102(3):278-80.
|
| Structure Identification | Int J Nanomedicine. 2009;4:321-33. Epub 2009 Dec 29.Optimization of the preparation process of vinblastine sulfate (VBLS)-loaded folate-conjugated bovine serum albumin (BSA) nanoparticles for tumor-targeted drug delivery using response surface methodology (RSM).[Pubmed: 20054435]Response surface methodology (RSM) was used to optimize the process of preparing bovine serum albumin (BSA) nanoparticles by desolvation, then the resulting BSA nanoparticles (BSANPs) were conjugated with folate to produce a drug carrier system that can specifically target tumors.
|

Vinblastine Sulfate Dilution Calculator

Vinblastine Sulfate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.1 mL | 5.5002 mL | 11.0004 mL | 22.0007 mL | 27.5009 mL |
| 5 mM | 0.22 mL | 1.1 mL | 2.2001 mL | 4.4001 mL | 5.5002 mL |
| 10 mM | 0.11 mL | 0.55 mL | 1.1 mL | 2.2001 mL | 2.7501 mL |
| 50 mM | 0.022 mL | 0.11 mL | 0.22 mL | 0.44 mL | 0.55 mL |
| 100 mM | 0.011 mL | 0.055 mL | 0.11 mL | 0.22 mL | 0.275 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: VIN showed EC50 values of 15 ug/ml, against P815 mastocytoma cells in-vitro[1]. Also supresses nAChR activity with IC50 of 8.9 μM. Vinblastine can inhibit the formation of microtubule, it also inhibit nAChR. It is a anticancer drug. More and more drug combination was investigated in clinical status. in vitro: At vinblastine concentrations of 0.5 uM and 2 uM, considerable inhibition of metabolic degradation of vinblastine was observed by competitive inhibitors of CYP3A4 (up to 60% inhibition), 2D6 (30%) and 2E1 (24%), while only a minor effect was observed for 2C9 (14%) and inhibitors of 1A2, 2C8 had no inhibitory effect on vinblastine metabolism. Vinblastine (0.5 and 2 uM) inhibited the metabolic capacity of CYP2C9 (up to 56%), 2C8 (36%), 2D6 (22%) and 3A4-mediated nifedipine oxidation (99%), while 3A4-mediated testosterone 6-beta-hydroxylation (max. 16%) as well as 1A2 and 2E1 remained unaffected[3]. in vivo: In combined intraperitoneal injection with vinblastine (200 micrograms kg-1) into P388/ADR-bearing mice, NA-382 in a suspension form (10 mg kg-1) prolonged the life-span of the mice near to that of P388/S-bearing mice treated with vinblastine alone, but verapamil even at the maximum tolerated dosage (30 mg kg-1) barely affected the in-vivo antitumour effect of vinblastine[2]. Clinical trial: Combination Chemotherapy With or Without Interleukin-2 and Interferon Alfa in Treating Patients With Metastatic Melanoma . Phase 3 Clinical
- Protoveratrine A
Catalog No.:BCN5346
CAS No.:143-57-7
- Lauric acid
Catalog No.:BCN2635
CAS No.:143-07-7
- Salvianolic acid D
Catalog No.:BCN2369
CAS No.:142998-47-8
- Salvianolic acid E
Catalog No.:BCN8194
CAS No.:142998-46-7
- Fmoc-D-Ala(3-pyridyl)-OH.HCl
Catalog No.:BCC3324
CAS No.:142994-45-4
- Fmoc-D-Phe(4-Cl)-OH
Catalog No.:BCC3177
CAS No.:142994-19-2
- UNC2025
Catalog No.:BCC8062
CAS No.:1429881-91-3
- HPOB
Catalog No.:BCC5574
CAS No.:1429651-50-2
- Triptoquinone A
Catalog No.:BCN6781
CAS No.:142950-86-5
- Triptoquinone B
Catalog No.:BCN6238
CAS No.:142937-50-6
- Mutant IDH1 inhibitor
Catalog No.:BCC4144
CAS No.:1429180-08-4
- OXF BD 02
Catalog No.:BCC5598
CAS No.:1429129-68-9
- ML216
Catalog No.:BCC8061
CAS No.:1430213-30-1
- 1alpha-Hydroxy VD4
Catalog No.:BCC1300
CAS No.:143032-85-3
- Ethyl 3,4-dicaffeoylquinate
Catalog No.:BCN8004
CAS No.:143051-73-4
- Phytic acid sodium salt hydrate
Catalog No.:BCN1283
CAS No.:14306-25-3
- BQ-3020
Catalog No.:BCC5728
CAS No.:143113-45-5
- Neotuberostemonine
Catalog No.:BCN6239
CAS No.:143120-46-1
- GSK-LSD1 2HCl
Catalog No.:BCC5647
CAS No.:1431368-48-7
- BMX-IN-1
Catalog No.:BCC1434
CAS No.:1431525-23-3
- H-D-Ala-OMe.HCl
Catalog No.:BCC3199
CAS No.:14316-06-4
- UNC1999
Catalog No.:BCC4552
CAS No.:1431612-23-5
- CAL-130
Catalog No.:BCC1440
CAS No.:1431697-74-3
- CAL-130 Hydrochloride
Catalog No.:BCC1441
CAS No.:1431697-78-7
Effect of vinblastine sulfate on gamma-radiation-induced DNA single-strand breaks in murine tissues.[Pubmed:12694742]
Mutat Res. 2003 Apr 20;536(1-2):15-25.
The effect of Vinblastine Sulfate on gamma-radiation-induced DNA strand breaks in different tissues of tumour bearing mice, was studied by single-cell gel electrophoresis. Intraperitonial administration of different doses (0.25-2.0mg/kg body weight) of Vinblastine Sulfate 30 min prior to 4 Gy gamma-radiation exposure showed a dose-dependent decrease in the yield of DNA strand breaks in murine fibrosarcoma, blood leukocytes and bone marrow cells. The dose-dependent protection of cellular DNA against radiation-induced strand breaks as evidenced from comet tail length, tail moment and percent DNA in the tail, was more pronounced in bone marrow cells than in the cells of the tumor fibrosarcoma. In fibrosarcoma cells, the decrease in comet tail length, tail moment and percent DNA in the tail was detected at lower doses of Vinblastine Sulfate administration and these parameters were not significantly altered at higher doses, from that of the control irradiated. From this study, it appears that in addition to anticancer activity, Vinblastine Sulfate could offer protection to the normal tissues against gamma-radiation-induced DNA strand breaks.
Anticancer drug vinblastine sulphate induces transient morphological changes on the olfactory mucosa of the rabbit.[Pubmed:22443492]
Anat Histol Embryol. 2012 Oct;41(5):374-87.
Vinblastine sulphate (VBS) is an anticancer drug that acts by disrupting microtubule dynamics of highly mitotic tissue cells. The consequences of VBS on the olfactory mucosa (OM), a tissue with high mitotic numbers, are not clearly understood. We used qualitative and quantitative methods to determine the structural changes that may be produced on the rabbit OM by VBS. Following a single dose (0.31 mg/kg) of this drug, the structure of the mucosa was greatly altered on the first 3-5 days. The alteration was characterized by disarrangement of the normal layering of nuclei of the epithelia, degeneration of axonal bundles, occurrence of blood vessels within the bundles, localized death of cells of Bowman's glands and glandular degeneration. Surprisingly on or after day 7 and progressively to day 15 post-exposure, the OM was observed to regenerate and acquire normal morphology, and the vessels disappeared from the bundles. Relative to control values, bundle diameters, olfactory cell densities and cilia numbers decreased to as low as 53.1, 75.2 and 71.4%, respectively, on day 5. Volume density for the bundles, which was 28.6% in controls, decreased to a lowest value of 16.8% on day 5. In contrast, the volume density for the blood vessels was significantly lower in controls (19.9%) than in treated animals at day 2 (25.8%), day 3 (34.3%) and day 5 (31.5%). These findings suggest that the changes induced on the rabbit OM by VBS are transient and that regenerative recovery leads to the restoration of the normal structure of the mucosa.
A comparison of nitrogen mustard and vinblastine sulfate in the treatment of patients with Hodgkin's disease.[Pubmed:5414540]
Can Med Assoc J. 1970 Feb 14;102(3):278-80.
In a crossover study the effectiveness of intermittent maintenance doses of nitrogen mustard was compared to that of Vinblastine Sulfate in the treatment of 61 patients with advanced Hodgkin's disease. Forty-five of the patients had had previous radiation therapy. Nine of 29 patients who received nitrogen mustard as the first drug had a complete response and five had a partial response. The comparative results in 32 patients receiving Vinblastine Sulfate first were nine complete responses and 13 partial responses. The median duration of the complete responses to each drug was 43 weeks. The partial responses were of shorter duration. When the second drug was given in adequate doses, almost as many patients responded with a similar median duration of response.It is concluded that nitrogen mustard and Vinblastine Sulfate are equally effective single agents in the treatment of patients with advanced Hodgkin's disease and that patient preference would favour Vinblastine Sulfate because of its negligible side effects.
Optimization of the preparation process of vinblastine sulfate (VBLS)-loaded folate-conjugated bovine serum albumin (BSA) nanoparticles for tumor-targeted drug delivery using response surface methodology (RSM).[Pubmed:20054435]
Int J Nanomedicine. 2009;4:321-33. Epub 2009 Dec 29.
Response surface methodology (RSM) was used to optimize the process of preparing bovine serum albumin (BSA) nanoparticles by desolvation, then the resulting BSA nanoparticles (BSANPs) were conjugated with folate to produce a drug carrier system that can specifically target tumors. The anticancer drug, Vinblastine Sulfate (VBLS), was loaded to this tumor-specific drug carrier system for the purpose of overcoming the nonspecific targeting characteristics and side effects of the drug. A central composite design was applied for modeling the process, which was composed of four independent variables, namely BSA concentration, the rate of adding ethanol (ethanol rate), ethanol amount, and the degree of crosslinking. The mean particle size and residual amino groups of the BSANPs were chosen as response variables. The interactive effects of the four independent variables on the response variables were studied. The characteristics of the nanoparticles; such as amount of folate conjugation, drug entrapment efficiency, drug-loading efficiency, surface morphology and release kinetics in vitro were investigated. Optimum conditions for preparing desired BSANPs, with a mean particle size of 156.6 nm and residual amino groups of 668.973 nM/mg, were obtained. The resulting folate-conjugated BSANPs (FA-BSANPs) showed a drug entrapment efficiency of 84.83% and drug-loading efficiency of 42.37%, respectively, and the amount of folate conjugation was 383.996 microM/g BSANPs. The results of this study indicate that using FA-BSANPs as a drug carrier system could be effective in targeting VBLS-sensitive tumors in the future.
The chemotherapeutic agent paclitaxel inhibits autophagy through two distinct mechanisms that regulate apoptosis.[Pubmed:22430212]
Oncogene. 2013 Feb 7;32(6):736-46.
Anti-mitotic agents such as paclitaxel and docetaxel are widely used for the treatment of breast, ovarian and lung cancers. Although paclitaxel induces apoptosis, this drug also modulates autophagy. How autophagy affects paclitaxel activity, is unclear. We discovered that paclitaxel inhibited autophagy through two distinct mechanisms dependent on cell cycle stage. In mitotic cells, paclitaxel blocked activation of the class III phosphatidyl inositol 3 kinase, Vps34, a critical initiator of autophagosome formation. In non-mitotic paclitaxel-treated cells, autophagosomes were generated but their movement and maturation was inhibited. Chemically or genetically blocking autophagosome formation diminished paclitaxel-induced cell death suggesting that autophagosome accumulation sensitized cells to paclitaxel toxicity. In line with these observations, we identified that primary breast tumors that expressed diminished levels of autophagy-initiating genes were resistant to taxane therapy, identifying possible mechanisms and prognostic markers of clinical chemotherapeutic resistance.
Localization of critical histidyl residues required for vinblastine-induced tubulin polymerization and for microtubule assembly.[Pubmed:9813016]
J Biol Chem. 1998 Nov 20;273(47):31131-7.
Vinblastine-induced tubulin polymerization is electrostatically regulated and shows pH dependence with a pI approximately 7.0 suggesting the involvement of histidyl residues. Modification of histidyl residues of tubulin with diethylpyrocarbonate (DEPC) at a mole ratio of 0.74 (DEPC/total His residues) for 3 min at 25 degreesC completely inhibited vinblastine-induced polymerization with little effect on microtubule assembly. Under these conditions DEPC reacts only with histidyl residues. For complete inhibition two histidyl residues have to be modified. Demodification of the carboxyethyl histidyl derivatives by hydroxylamine led to nearly complete recovery of polymerization competence. Labeling with [14C]DEPC localized both of these histidyl residues on beta-tubulin at beta227 and beta264. Similarly, tubulin modification with DEPC for longer times (8 min) resulted in complete inhibition of microtubule assembly, at which time approximately 4 histidyl residues had been modified. This inhibition by DEPC was also reversed by hydroxylamine. The third histidyl residue was found on alpha-tubulin at alpha88. Thus, two charged histidyl residues are obligatorily involved in vinblastine-induced polymerization, whereas a different histidyl residue on a different tubulin monomer is involved in microtubule assembly.
Microtubule antagonists activate programmed cell death (apoptosis) in cultured rat hepatocytes.[Pubmed:8362985]
Am J Pathol. 1993 Sep;143(3):918-25.
We investigated the mechanism of lethal injury following the disruption of microtubules in cultured hepatocytes treated with vinblastine (VBL) or colchicine (COL). These agents kill hepatocytes by a process readily distinguished from two well-known pathways that lead to a loss of viability, namely, oxidative stress and inhibition of mitochondrial electron transport. Cell killing with VBL and COL was accompanied by fragmentation of DNA. Both the loss of viability and the fragmentation of DNA were prevented by the inhibition of protein synthesis within 6 hours following exposure to VBL or COL. Cell death and the fragmentation of DNA were also prevented when Ca2+ was removed from the culture medium. By contrast, the inhibition of protein kinase C prevented cell killing by VBL or COL, but did not alter the extent of DNA fragmentation. The requirements here for protein synthesis, extracellular Ca2+, and protein kinase C activity define a model of apoptosis, or programmed cell death, that seems to involve mechanisms that can be dissociated from the fragmentation of DNA.
Comparison of the effects of vinblastine, vincristine, vindesine, and vinepidine on microtubule dynamics and cell proliferation in vitro.[Pubmed:3986806]
Cancer Res. 1985 Jun;45(6):2741-7.
Vinepidine, a new derivative of vincristine, and three clinically used Catharanthus derivatives, vinblastine, vincristine, and vindesine, were examined for their abilities to inhibit net tubulin addition at the assembly ends of bovine brain microtubules at steady state. Although all four derivatives were generally similar in potency, their relative abilities to inhibit tubulin addition were distinguishable. Vinepidine and vincristine were the most potent derivatives (Ki, 0.079 +/- 0.018 (SD) microM and 0.085 +/- 0.013 microM, respectively), followed by vindesine (Ki, 0.110 +/- 0.007 microM) and vinblastine (Ki, 0.178 +/- 0.025 microM). In contrast to their relative abilities to inhibit microtubule assembly in vitro, vinblastine and its derivative, vindesine, were generally more potent than vincristine and vinepidine in inhibiting cell proliferation in culture. Vinblastine was nine times more potent than the weakest derivative, vinepidine, in B16 melanoma cells. In L-cells, vinblastine completely inhibited growth at 40 nM, whereas vincristine and vindesine caused about 25% inhibition, and vinepidine was inactive. When B16 melanoma cells were treated with drug before being injected into mice, retardation of tumor growth was best achieved with vindesine, one of the weaker of the four derivatives in vitro. The results demonstrate that chemical differences among the Catharanthus derivatives, which affect to small extents the abilities of the derivatives to inhibit microtubule assembly in vitro, result in significant differences in the order and the magnitude of the abilities of the drugs to inhibit cell growth.


