UNC1999EZH2 inhibitor CAS# 1431612-23-5 |
- 3-Deazaneplanocin,DZNep
Catalog No.:BCC1129
CAS No.:102052-95-9
- EPZ005687
Catalog No.:BCC2219
CAS No.:1396772-26-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1431612-23-5 | SDF | Download SDF |
| PubChem ID | 72551585 | Appearance | Powder |
| Formula | C33H43N7O2 | M.Wt | 569.74 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 125 mg/mL (219.40 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
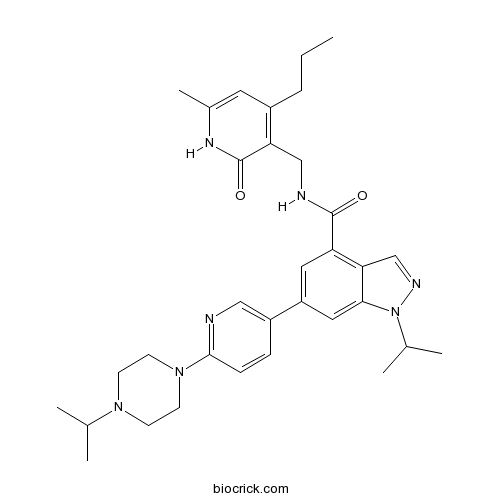
| Chemical Name | N-[(6-methyl-2-oxo-4-propyl-1H-pyridin-3-yl)methyl]-1-propan-2-yl-6-[6-(4-propan-2-ylpiperazin-1-yl)pyridin-3-yl]indazole-4-carboxamide | ||
| SMILES | CCCC1=C(C(=O)NC(=C1)C)CNC(=O)C2=C3C=NN(C3=CC(=C2)C4=CN=C(C=C4)N5CCN(CC5)C(C)C)C(C)C | ||
| Standard InChIKey | DPJNKUOXBZSZAI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C33H43N7O2/c1-7-8-24-15-23(6)37-33(42)28(24)19-35-32(41)27-16-26(17-30-29(27)20-36-40(30)22(4)5)25-9-10-31(34-18-25)39-13-11-38(12-14-39)21(2)3/h9-10,15-18,20-22H,7-8,11-14,19H2,1-6H3,(H,35,41)(H,37,42) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and SAM-competitive EZH2/EZH1 lysine methyltransferase inhibitor (IC50 values are 2 and 45 nM respectively). Selective for EZH2/EZH1 over a panel of other methyltransferases and non-epigenetic targets. Reduces H3K27me3 levels in vitro. Prolongs survival of MLL-AF9 bearing mice. Orally bioavailable. | |||||

UNC1999 Dilution Calculator

UNC1999 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7552 mL | 8.7759 mL | 17.5519 mL | 35.1037 mL | 43.8797 mL |
| 5 mM | 0.351 mL | 1.7552 mL | 3.5104 mL | 7.0207 mL | 8.7759 mL |
| 10 mM | 0.1755 mL | 0.8776 mL | 1.7552 mL | 3.5104 mL | 4.388 mL |
| 50 mM | 0.0351 mL | 0.1755 mL | 0.351 mL | 0.7021 mL | 0.8776 mL |
| 100 mM | 0.0176 mL | 0.0878 mL | 0.1755 mL | 0.351 mL | 0.4388 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
UNC1999 is an orally bioavailable inhibitor of EZH2 and EZH1 with IC50 values of [1].
UNC1999 binds to the EZH2 SAM binding site of EZH2 and competes with the cofactor SAM with a Ki of 4.6nM. It does not compete with the H3 peptide substrate. UNC1999 also has potency for mutant EZH2, including Y641N and Y641F mutants. Besides EZH2, UNC1999 is effective for EZH1 with IC50 value of 45nM. UNC1999 is shown to be more than 200-fold selective for EZH2 over a broad range of non-epigenetic targets, including kinases, GPCRs, transporters and ion channels [1].
EZH2 catalyzes methylation of H3K27, producing H3K27me3. H3K27me3 is a transcriptionally repressive post-translational modification. As an inhibitor of EZH2, UNC1999 reduces H3K27me3 with IC50 value of 124nM in the In-Cell Western assay. Additionally, UNC1999 can inhibit cell proliferation in a DLBCL cell line harboring the Y641N mutant with EC50 value of 633nM. Moreover, UNC1999 is reported to be orally bioavailable in mice [1].
References:
[1] Konze KD, Ma A, Li F, Barsyte-Lovejoy D, Parton T, Macnevin CJ, Liu F, Gao C, Huang XP, Kuznetsova E, Rougie M, Jiang A, Pattenden SG, Norris JL, James LI, Roth BL, Brown PJ, Frye SV, Arrowsmith CH, Hahn KM, Wang GG, Vedadi M, Jin J. An orally bioavailable chemical probe of the Lysine Methyltransferases EZH2 and EZH1. ACS Chem Biol. 2013;8(6):1324-34.
- H-D-Ala-OMe.HCl
Catalog No.:BCC3199
CAS No.:14316-06-4
- BMX-IN-1
Catalog No.:BCC1434
CAS No.:1431525-23-3
- GSK-LSD1 2HCl
Catalog No.:BCC5647
CAS No.:1431368-48-7
- Neotuberostemonine
Catalog No.:BCN6239
CAS No.:143120-46-1
- BQ-3020
Catalog No.:BCC5728
CAS No.:143113-45-5
- Phytic acid sodium salt hydrate
Catalog No.:BCN1283
CAS No.:14306-25-3
- Ethyl 3,4-dicaffeoylquinate
Catalog No.:BCN8004
CAS No.:143051-73-4
- 1alpha-Hydroxy VD4
Catalog No.:BCC1300
CAS No.:143032-85-3
- ML216
Catalog No.:BCC8061
CAS No.:1430213-30-1
- Vinblastine Sulfate
Catalog No.:BCN2292
CAS No.:143-67-9
- Protoveratrine A
Catalog No.:BCN5346
CAS No.:143-57-7
- Lauric acid
Catalog No.:BCN2635
CAS No.:143-07-7
- CAL-130
Catalog No.:BCC1440
CAS No.:1431697-74-3
- CAL-130 Hydrochloride
Catalog No.:BCC1441
CAS No.:1431697-78-7
- gamma-secretase modulator 3
Catalog No.:BCC1585
CAS No.:1431697-84-5
- AT7519 trifluoroacetate
Catalog No.:BCC1377
CAS No.:1431697-85-6
- AT7867 dihydrochloride
Catalog No.:BCC1378
CAS No.:1431697-86-7
- OTSSP167
Catalog No.:BCC4314
CAS No.:1431697-89-0
- SB-408124 Hydrochloride
Catalog No.:BCC1929
CAS No.:1431697-90-3
- CCT241533 hydrochloride
Catalog No.:BCC1463
CAS No.:1431697-96-9
- KN-92 hydrochloride
Catalog No.:BCC1681
CAS No.:1431698-47-3
- (S)-Tedizolid
Catalog No.:BCC1294
CAS No.:1431699-67-0
- WEHI-539
Catalog No.:BCC2055
CAS No.:1431866-33-9
- K02288
Catalog No.:BCC5084
CAS No.:1431985-92-0
(18)F-Labeled PET Probe Targeting Enhancer of Zeste Homologue 2 (EZH2) for Cancer Imaging.[Pubmed:30891136]
ACS Med Chem Lett. 2019 Feb 27;10(3):334-340.
The enzyme enhancer of zeste homologue 2 (EZH2) plays a catalytic role in histone methylation (H3K27me3), one of the epigenetic modifications that is dysregulated in cancer. The development of a positron emission tomography (PET) imaging agent targeting EZH2 has the potential to provide a method of stratifying patients for epigenetic therapies. In this study, we designed and synthesized a series of fluoroethyl analogs based upon the structure of EZH2 inhibitors UNC1999 and EPZ6438. Among the candidate compounds, 20b exhibited a high binding affinity to EZH2 (IC50 = 6 nM) with selectivity versus EZH1 (IC50 = 200 nM) by SAM competition assay, and furthermore, EZH2 inhibition was demonstrated in the pancreatic cancer cell line PANC-1 (IC50 = 9.8 nM). [(18)F]20b was synthesized successfully and showed 5-fold higher uptake in PANC-1 cells than in MCF-7 cells. MicroPET imaging in a PANC-1 cell xenograft mouse model indicates that [(18)F]20b has specific binding to EZH2, which was identified by ex vivo Western blot analysis of the tumor tissue.
Six Years (2012-2018) of Researches on Catalytic EZH2 Inhibitors: The Boom of the 2-Pyridone Compounds.[Pubmed:30338896]
Chem Rec. 2018 Dec;18(12):1818-1832.
Enhancer of zeste homolog 2 (EZH2), the catalytic subunit of the Polycomb repressive complex 2 (PRC2), catalyzes the methylation of lysine 27 of histone H3 (H3K27) up to its trimethylated form (H3K27me), inducing by this way block of transcription and gene silencing. High levels of H3K27me3 have been found in both hematological malignancies and solid cancers, due to EZH2 overexpression and/or EZH2 mutation. From 2012, a number of highly potent and selective catalytic inhibitors of EZH2 have been reported, almost all bearing a 2-pyridone group in their structure. Typically, 2-pyridone inhibitors are selective for EZH2 over other methyltransferases, and some of them are specific for EZH2 over EZH1, others behave as dual EZH2/EZH1 inhibitors. The 2-pyridone moiety was crucial for the enzyme inhibition, as revealed later by crystallographic studies because it occupies partially the site for the co-substrate SAM (or the by-product, SAH) in the binding pocket of the enzyme, accounting for the SAM-competitive mechanism of action displayed by all the 2-pyridone inhibitors. The 2-pyridone warhead is linked to a support substructure, that can be either a bicyclic heteroaromatic ring (such as indazole, see for instance EPZ005687 and UNC1999, or indole, see for instance GSK126, EI1, and the more recent CPI-1205) or a simple monocyclic (hetero) aromatic ring (tazemetostat, MC3629, (R)-OR-S1/2), eventually annulated with the amide chain carrying the 2-pyridone group (3,4-dihydroisoquinoline-1(2H)-ones). Different substitutions at the support moiety influence the pharmacokinetics and pharmacodynamics of the compounds as well as their water solubility. In cancer diseases, the first reported 2-pyridone inhibitors displayed high antiproliferative effects in vitro and in vivo in lymphomas characterized by mutant EZH2 (such as Y641N), but the most recent compounds exert their anticancer activity against tumors with wild-type EZH2 as well. The dual EZH2/1 inhibitors have been recently reported to be more effective than EZH2 selective inhibitors in specific leukemias including leukemias cancer stem cells.
Overexpression of EZH2 in conjunctival melanoma offers a new therapeutic target.[Pubmed:29732557]
J Pathol. 2018 Aug;245(4):433-444.
Malignant melanoma of the conjunctiva (CM) is an uncommon but potentially deadly disorder. Many malignancies show an increased activity of the epigenetic modifier enhancer of zeste homolog 2 (EZH2). We studied whether EZH2 is expressed in CM, and whether it may be a target for therapy in this malignancy. Immunohistochemical analysis showed that EZH2 protein expression was absent in normal conjunctival melanocytes and primary acquired melanosis, while EZH2 was highly expressed in 13 (50%) of 26 primary CM and seven (88%) of eight lymph node metastases. Increased expression was positively associated with tumour thickness (p =0.03). Next, we targeted EZH2 with specific inhibitors (GSK503 and UNC1999) or depleted EZH2 by stable shRNA knockdown in three primary CM cell lines. Both pharmacological and genetic inactivation of EZH2 inhibited cell growth and colony formation and influenced EZH2-mediated gene transcription and cell cycle profile in vitro. The tumour suppressor gene p21/CDKN1A was especially upregulated in CM cells after EZH2 knockdown in CM cells. Additionally, the potency of GSK503 against CM cells was monitored in zebrafish xenografts. GSK503 profoundly attenuated tumour growth in CM xenografts at a well-tolerated concentration. Our results indicate that elevated levels of EZH2 are relevant to CM tumourigenesis and progression, and that EZH2 may become a potential therapeutic target for patients with CM. (c) 2018 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Mechanisms of resistance to EZH2 inhibitors in diffuse large B-cell lymphomas.[Pubmed:29572378]
Blood. 2018 May 10;131(19):2125-2137.
Resistance to targeted therapies has become increasingly prevalent. We noted that resistance to different targeted therapies occurs by largely common mechanisms. In this study, we used this information for identifying the mechanisms of resistance to enhancer of zeste homolog 2 (EZH2) inhibitors in diffuse large B-cell lymphoma (DLBCL) harboring EZH2 mutations. We discovered that EZH2 inhibitor-resistant DLBCL cells showed activation of the insulin-like growth factor 1 receptor (IGF-1R), MEK, and phosphoinositide-3-kinase (PI3K) pathways. Constitutive activation of IGF-1R, MEK, or PI3K pathways was sufficient to confer resistance to EZH2 inhibitors in DLBCL. The activation of the PI3K/AKT and MAPK pathways decreased TNFSF10 and BAD expression through a FOXO3-dependent mechanism, which was required for the antitumor effects of EZH2i GSK126. We also identified multiple acquired mutations in EZH2 inhibitor-resistant DLBCL cell lines. These mutations independently conferred resistance to EZH2 inhibitors. Mechanistically, cellular thermal shift assays revealed that the acquired EZH2 mutations that confer resistance to EZH2 inhibitors prevent EZH2 inhibitor binding to the EZH2 mutants. Notably, EZH2 inhibitor GSK126- and EPZ-6438-resistant DLBCL cells remained sensitive to the EZH2 inhibitor UNC1999 and embryonic ectoderm development protein inhibitor EED226, which provides an opportunity to treat DLBCLs that are resistant to these drugs. Collectively, our results underpin the importance for developing a unified approach for forestalling drug resistance by prospectively considering lessons learned from the use of different targeted therapeutic agents.
EZH2 inhibits autophagic cell death of aortic vascular smooth muscle cells to affect aortic dissection.[Pubmed:29416002]
Cell Death Dis. 2018 Feb 7;9(2):180.
Enhancer of zeste homolog 2 (EZH2), a methyltransferase that di- and tri-methylates lysine-27 of histone H3, largely functions as a transcriptional repressor, and plays a critical role in various kinds of cancers. Here we report a novel function of EZH2 in regulating autophagic cell death (ACD) of vascular smooth muscle cells (VSMCs) that affect aortic dissection (AD). Inhibition of EZH2 activity by UNC1999 or knockdown EZH2 resulted in VSMC loss, while overexpression of EZH2 facilitated VSMC growth, and these effects of EZH2 on VSMCs were independent of proliferation and apoptosis. Interestingly, more autophagic vacuoles and increased LC3II protein levels were identified in VSMCs with EZH2 inhibition or deficiency. Moreover, when compared with counterparts, chloroquine alone, or chloroquine with rapamycin treatment led to more LC3II accumulation in EZH2 inhibited or knockdown VSMCs, which indicated that EZH2 negatively regulated autophagosome formation. In conjunction to this, ATG5 and ATG7 protein levels were remarkably increased in EZH2 inhibited or deficient VSMCs, and ATG5 or ATG7 knockdown virtually rescued VSMC loss induced by EZH2 inhibition or knockdown. In addition, we found that the MEK-ERK1/2 signaling pathway, but not AMPKalpha, mTOR, or AKT pathway, is responsible for the impact of EZH2 on ACD of VSMCs. Additionally, the adverse effects of EZH2 inhibition or knockdown on VSMCs were largely reversed by PD98059, an inhibitor of MEK1. More importantly, decreased EZH2 expression levels in the aortic wall of patients with AD indicated its contribution to VSMC loss and AD occurrence. Overall, these findings revealed that EZH2 affects ACD of VSMCs and the pathologic process of AD via regulating ATG5 and ATG7 expression and MEK-ERK1/2 signaling. Our hitherto unrecognized findings indicate that EZH2 activation has therapeutic or preventive potential for AD.
The miR-125a and miR-320c are potential tumor suppressor microRNAs epigenetically silenced by the polycomb repressive complex 2 in multiple myeloma.[Pubmed:28664185]
RNA Dis. 2017;4(2).
We have previously presented the histone methyltransferase enhancer of zeste homolog 2 (EZH2) of the polycomb repressive complex 2 (PRC2) as a potential therapeutic target in Multiple Myeloma (MM). In a recent article in Oncotarget by Alzrigat. et al. 2017, we have reported on the novel finding that EZH2 inhibition using the highly selective inhibitor of EZH2 enzymatic activity, UNC1999, reactivated the expression of microRNA genes previously reported to be underexpressed in MM. Among these, we have identified miR-125a-3p and miR-320c as potential tumor suppressor microRNAs as they were predicted to target MM-associated oncogenes; IRF-4, XBP-1 and BLIMP-1. We also found EZH2 inhibition to reactivate the expression of miR-494, a previously reported regulator of the c-MYC oncogene. In addition, we could report that EZH2 inhibition downregulated the expression of a few well described oncogenic microRNAs in MM. The data from our recent article are here highlighted as it shed a new light onto the oncogenic function of the PRC2 in MM. These data further strengthen the notion that the PRC2 complex may be of potential therapeutic interest.
Dual Inhibition of EZH2 and EZH1 Sensitizes PRC2-Dependent Tumors to Proteasome Inhibition.[Pubmed:28490465]
Clin Cancer Res. 2017 Aug 15;23(16):4817-4830.
Purpose: EZH2 and EZH1, the catalytic components of polycomb repressive complex 2 (PRC2), trigger trimethylation of H3K27 (H3K27me3) to repress the transcription of target genes and are implicated in the pathogenesis of various cancers including multiple myeloma and prostate cancer. Here, we investigated the preclinical effects of UNC1999, a dual inhibitor of EZH2 and EZH1, in combination with proteasome inhibitors on multiple myeloma and prostate cancer.Experimental Design:In vitro and in vivo efficacy of UNC1999 and the combination with proteasome inhibitors was evaluated in multiple myeloma cell lines, primary patient cells, and in a xenograft model. RNA-seq and ChIP-seq were performed to uncover the targets of UNC1999 in multiple myeloma. The efficacy of the combination therapy was validated in prostate cancer cell lines.Results: Proteasome inhibitors repressed EZH2 transcription via abrogation of the RB-E2F pathway, thereby sensitizing EZH2-dependent multiple myeloma cells to EZH1 inhibition by UNC1999. Correspondingly, combination of proteasome inhibitors with UNC1999, but not with an EZH2-specific inhibitor, induced synergistic antimyeloma activity in vitro Bortezomib combined with UNC1999 remarkably inhibited the growth of myeloma cells in vivo Comprehensive analyses revealed several direct targets of UNC1999 including the tumor suppressor gene NR4A1 Derepression of NR4A1 by UNC1999 resulted in suppression of MYC, which was enhanced by the combination with bortezomib, suggesting the cooperative blockade of PRC2 function. Notably, this combination also exhibited strong synergy in prostate cancer cells.Conclusions: Our results identify dual inhibition of EZH2 and EZH1 together with proteasome inhibition as a promising epigenetics-based therapy for PRC2-dependent cancers. Clin Cancer Res; 23(16); 4817-30. (c)2017 AACR.
Multicellular Tumor Spheroids Combined with Mass Spectrometric Histone Analysis To Evaluate Epigenetic Drugs.[Pubmed:28194967]
Anal Chem. 2017 Mar 7;89(5):2773-2781.
Multicellular tumor spheroids (MCTS) are valuable in vitro tumor models frequently used to evaluate the penetration and efficacy of therapeutics. In this study, we evaluated potential differences in epigenetic markers, i.e., histone post-translational modifications (PTMs), in the layers of the HCT116 colon carcinoma MCTS. Cells were grown in agarose-coated 96 well plates, forming reproducible 1-mm-diameter MCTS. The MCTS were fractionated into three radially concentric portions, generating samples containing cells from the core, the mid and the external layers. Using mass spectrometry (MS)-based proteomics and EpiProfile, we quantified hundreds of histone peptides in different modified forms; by combining the results of all experiments, we quantified the abundance of 258 differently modified peptides, finding significant differences in their relative abundance across layers. Among these differences, we detected higher amounts of the repressive mark H3K27me3 in the external layers, compared to the core. We then evaluated the epigenetic response of MCTS following UNC1999 treatment, a drug targeting the enzymes that catalyze H3K27me3, namely, the polycomb repressive complex 2 (PRC2) subunits enhancer of zeste 1 (EZH1) and enhancer of zeste 2 (EZH2). UNC1999 treatment resulted in significant differences in MCTS diameter under drug treatment of varying duration. Using matrix-assisted laser desorption/ionization (MALDI) imaging, we determined that the drug penetrates the entire MCTS. Proteomic analysis revealed a decrease in abundance of H3K27me3, compared to the untreated sample, as expected. Interestingly, we observed a comparable growth curve for MCTS under constant drug treatment over 13 days with those treated for only 4 days at the beginning of their growth. We thus demonstrate that MS-based proteomics can define significant differences in histone PTM patterns in submillimetric layers of three-dimensional (3D) cultures. Moreover, we show that our model is suitable for monitoring drug localization and regulation of histone PTMs after drug treatment.
EZH2 inhibitors transcriptionally upregulate cytotoxic autophagy and cytoprotective unfolded protein response in human colorectal cancer cells.[Pubmed:27648357]
Am J Cancer Res. 2016 Aug 1;6(8):1661-80. eCollection 2016.
Enhancer of zeste homolog 2 (EZH2) has been emerged as novel anticancer target. Various EZH2 small-molecule inhibitors have been developed in recent years. A major class of EZH2 inhibitors are S-adenosyl-L-methionine (SAM)-competitive inhibitors, such as EPZ005687, EI1, GSK126, UNC1999 and GSK343. Autophagy, a physiological process of self-digestion, is involved in the turnover of proteins or intracellular organelles. It can serve as cytoprotective or cytotoxic function in cancer. Our previous study has found that UNC1999 and GSK343 are potent autophagy inducers. In this study, the underlying molecular mechanisms were further investigated. Our results showed that UNC1999 and GSK343 transcriptionally upregulated autophagy of human colorectal cancer (CRC) cells through inducing LC3B gene expression. Besides, UNC1999/GSK343-induced autophagy was partially dependent on ATG7 but independent to EZH2 inhibition. Microarray and PCR array analyses identified that UNC1999 and GSK343 also induced endoplasmic reticulum (ER) stress and unfolded protein response (UPR). UNC1999/GSK343-induced ER stress/UPR contributed to the survival of cancer cells, which was opposite to UNC1999/GSK343-induced autophagy that promoted cell death.
Structure-Activity Relationship Studies for Enhancer of Zeste Homologue 2 (EZH2) and Enhancer of Zeste Homologue 1 (EZH1) Inhibitors.[Pubmed:27468126]
J Med Chem. 2016 Aug 25;59(16):7617-33.
EZH2 or EZH1 (enhancer of zeste homologue 2 or 1) is the catalytic subunit of polycomb repressive complex 2 (PRC2) that catalyzes methylation of histone H3 lysine 27 (H3K27). PRC2 hyperactivity and/or hypertrimethylation of H3K27 are associated with numerous human cancers, therefore inhibition of PRC2 complex has emerged as a promising therapeutic approach. Recent studies have shown that EZH2 and EZH1 are not functionally redundant and inhibition of both EZH2 and EZH1 is necessary to block the progression of certain cancers such as mixed-lineage leukemia (MLL)-rearranged leukemias. Despite the significant advances in discovery of EZH2 inhibitors, there has not been a systematic structure-activity relationship (SAR) study to investigate the selectivity between EZH2 and EZH1 inhibition. Here, we report our SAR studies that focus on modifications to various regions of the EZH2/1 inhibitor UNC1999 (5) to investigate the impact of the structural changes on EZH2 and EZH1 inhibition and selectivity.
Small molecule epigenetic screen identifies novel EZH2 and HDAC inhibitors that target glioblastoma brain tumor-initiating cells.[Pubmed:27449082]
Oncotarget. 2016 Sep 13;7(37):59360-59376.
Glioblastoma (GBM) is the most lethal and aggressive adult brain tumor, requiring the development of efficacious therapeutics. Towards this goal, we screened five genetically distinct patient-derived brain-tumor initiating cell lines (BTIC) with a unique collection of small molecule epigenetic modulators from the Structural Genomics Consortium (SGC). We identified multiple hits that inhibited the growth of BTICs in vitro, and further evaluated the therapeutic potential of EZH2 and HDAC inhibitors due to the high relevance of these targets for GBM. We found that the novel SAM-competitive EZH2 inhibitor UNC1999 exhibited low micromolar cytotoxicity in vitro on a diverse collection of BTIC lines, synergized with dexamethasone (DEX) and suppressed tumor growth in vivo in combination with DEX. In addition, a unique brain-penetrant class I HDAC inhibitor exhibited cytotoxicity in vitro on a panel of BTIC lines and extended survival in combination with TMZ in an orthotopic BTIC model in vivo. Finally, a combination of EZH2 and HDAC inhibitors demonstrated synergy in vitro by augmenting apoptosis and increasing DNA damage. Our findings identify key epigenetic modulators in GBM that regulate BTIC growth and survival and highlight promising combination therapies.
Uropathogenic E. coli (UPEC) Infection Induces Proliferation through Enhancer of Zeste Homologue 2 (EZH2).[Pubmed:26964089]
PLoS One. 2016 Mar 10;11(3):e0149118.
UNLABELLED: Host-pathogen interactions can induce epigenetic changes in the host directly, as well as indirectly through secreted factors. Previously, uropathogenic Escherichia coli (UPEC) was shown to increase DNA methyltransferase activity and expression, which was associated with methylation-dependent alterations in the urothelial expression of CDKN2A. Here, we showed that paracrine factors from infected cells alter expression of another epigenetic writer, EZH2, coordinate with proliferation. Urothelial cells were inoculated with UPEC, UPEC derivatives, or vehicle (mock infection) at low moi, washed, then maintained in media with Gentamycin. Urothelial conditioned media (CM) and extracellular vesicles (EV) were isolated after the inoculations and used to treat naive urothelial cells. EZH2 increased with UPEC infection, inoculation-induced CM, and inoculation-induced EV vs. parallel stimulation derived from mock-inoculated urothelial cells. We found that infection also increased proliferation at one day post-infection, which was blocked by the EZH2 inhibitor UNC1999. Inhibition of demethylation at H3K27me3 had the opposite effect and augmented proliferation. CONCLUSION: Uropathogen-induced paracrine factors act epigenetically by altering expression of EZH2, which plays a key role in early host cell proliferative responses to infection.
Selective inhibition of EZH2 and EZH1 enzymatic activity by a small molecule suppresses MLL-rearranged leukemia.[Pubmed:25395428]
Blood. 2015 Jan 8;125(2):346-57.
Enhancer of zeste homolog 2 (EZH2) and related EZH1 control gene expression and promote tumorigenesis via methylating histone H3 at lysine 27 (H3K27). These methyltransferases are ideal therapeutic targets due to their frequent hyperactive mutations and overexpression found in cancer, including hematopoietic malignancies. Here, we characterized a set of small molecules that allow pharmacologic manipulation of EZH2 and EZH1, which include UNC1999, a selective inhibitor of both enzymes, and UNC2400, an inactive analog compound useful for assessment of off-target effect. UNC1999 suppresses global H3K27 trimethylation/dimethylation (H3K27me3/2) and inhibits growth of mixed lineage leukemia (MLL)-rearranged leukemia cells. UNC1999-induced transcriptome alterations overlap those following knockdown of embryonic ectoderm development, a common cofactor of EZH2 and EZH1, demonstrating UNC1999's on-target inhibition. Mechanistically, UNC1999 preferentially affects distal regulatory elements such as enhancers, leading to derepression of polycomb targets including Cdkn2a. Gene derepression correlates with a decrease in H3K27me3 and concurrent gain in H3K27 acetylation. UNC2400 does not induce such effects. Oral administration of UNC1999 prolongs survival of a well-defined murine leukemia model bearing MLL-AF9. Collectively, our study provides the detailed profiling for a set of chemicals to manipulate EZH2 and EZH1 and establishes specific enzymatic inhibition of polycomb repressive complex 2 (PRC2)-EZH2 and PRC2-EZH1 by small-molecule compounds as a novel therapeutics for MLL-rearranged leukemia.
An orally bioavailable chemical probe of the Lysine Methyltransferases EZH2 and EZH1.[Pubmed:23614352]
ACS Chem Biol. 2013;8(6):1324-34.
EZH2 or EZH1 is the catalytic subunit of the polycomb repressive complex 2 that catalyzes methylation of histone H3 lysine 27 (H3K27). The trimethylation of H3K27 (H3K27me3) is a transcriptionally repressive post-translational modification. Overexpression of EZH2 and hypertrimethylation of H3K27 have been implicated in a number of cancers. Several selective inhibitors of EZH2 have been reported recently. Herein we disclose UNC1999, the first orally bioavailable inhibitor that has high in vitro potency for wild-type and mutant EZH2 as well as EZH1, a closely related H3K27 methyltransferase that shares 96% sequence identity with EZH2 in their respective catalytic domains. UNC1999 was highly selective for EZH2 and EZH1 over a broad range of epigenetic and non-epigenetic targets, competitive with the cofactor SAM and non-competitive with the peptide substrate. This inhibitor potently reduced H3K27me3 levels in cells and selectively killed diffused large B cell lymphoma cell lines harboring the EZH2(Y641N) mutant. Importantly, UNC1999 was orally bioavailable in mice, making this inhibitor a valuable tool for investigating the role of EZH2 and EZH1 in chronic animal studies. We also designed and synthesized UNC2400, a close analogue of UNC1999 with potency >1,000-fold lower than that of UNC1999 as a negative control for cell-based studies. Finally, we created a biotin-tagged UNC1999 (UNC2399), which enriched EZH2 in pull-down studies, and a UNC1999-dye conjugate (UNC2239) for co-localization studies with EZH2 in live cells. Taken together, these compounds represent a set of useful tools for the biomedical community to investigate the role of EZH2 and EZH1 in health and disease.


