(S)-TedizolidAntibacterial oxazolidinone against gram-positive species CAS# 1431699-67-0 |
- FLAG tag Peptide
Catalog No.:BCC2562
CAS No.:98849-88-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1431699-67-0 | SDF | Download SDF |
| PubChem ID | 71576658 | Appearance | Powder |
| Formula | C17H15FN6O3 | M.Wt | 370.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | (S)-TR 700; (S)-DA 7157 | ||
| Solubility | DMSO : ≥ 100 mg/mL (270.02 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (5S)-3-[3-fluoro-4-[6-(2-methyltetrazol-5-yl)pyridin-3-yl]phenyl]-5-(hydroxymethyl)-1,3-oxazolidin-2-one | ||
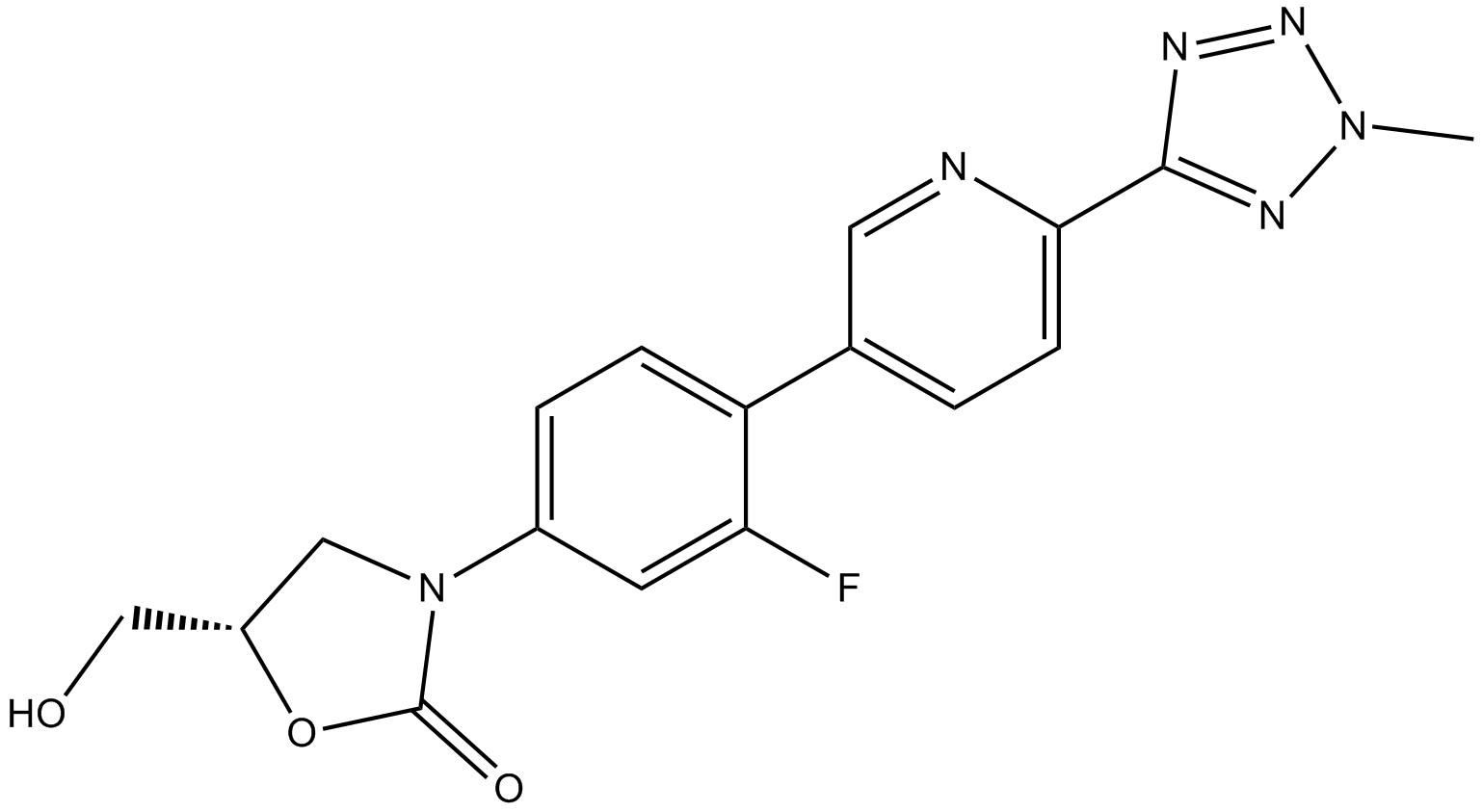
| SMILES | CN1N=C(N=N1)C2=NC=C(C=C2)C3=C(C=C(C=C3)N4CC(OC4=O)CO)F | ||
| Standard InChIKey | XFALPSLJIHVRKE-LBPRGKRZSA-N | ||
| Standard InChI | InChI=1S/C17H15FN6O3/c1-23-21-16(20-22-23)15-5-2-10(7-19-15)13-4-3-11(6-14(13)18)24-8-12(9-25)27-17(24)26/h2-7,12,25H,8-9H2,1H3/t12-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | (S)-Tedizolid is the S-enantiomer of Tedizolid. Tedizolid is a novel oxazolidinone with activity against Gram-positive pathogens. (S)-Tedizolid is the less active isomer. |

(S)-Tedizolid Dilution Calculator

(S)-Tedizolid Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Torezolid (also known as TR-701 and now tedizolid) is an oxazolidinone drug in phase-II clinical trials for complicated skin and skin-structure infections (cSSSI), including those caused by Methicillin-resistant Staphylococcus aureus (MRSA). From Wikipedia
- KN-92 hydrochloride
Catalog No.:BCC1681
CAS No.:1431698-47-3
- CCT241533 hydrochloride
Catalog No.:BCC1463
CAS No.:1431697-96-9
- SB-408124 Hydrochloride
Catalog No.:BCC1929
CAS No.:1431697-90-3
- OTSSP167
Catalog No.:BCC4314
CAS No.:1431697-89-0
- AT7867 dihydrochloride
Catalog No.:BCC1378
CAS No.:1431697-86-7
- AT7519 trifluoroacetate
Catalog No.:BCC1377
CAS No.:1431697-85-6
- gamma-secretase modulator 3
Catalog No.:BCC1585
CAS No.:1431697-84-5
- CAL-130 Hydrochloride
Catalog No.:BCC1441
CAS No.:1431697-78-7
- CAL-130
Catalog No.:BCC1440
CAS No.:1431697-74-3
- UNC1999
Catalog No.:BCC4552
CAS No.:1431612-23-5
- H-D-Ala-OMe.HCl
Catalog No.:BCC3199
CAS No.:14316-06-4
- BMX-IN-1
Catalog No.:BCC1434
CAS No.:1431525-23-3
- WEHI-539
Catalog No.:BCC2055
CAS No.:1431866-33-9
- K02288
Catalog No.:BCC5084
CAS No.:1431985-92-0
- 3'-O-Galloylmyricitrin
Catalog No.:BCN8268
CAS No.:143202-36-2
- WAY 267464 dihydrochloride
Catalog No.:BCC7813
CAS No.:1432043-31-6
- Nyasicol 1,2-acetonide
Catalog No.:BCN6879
CAS No.:1432057-64-1
- Catalponol methylthiomethyl ether
Catalog No.:BCC8905
CAS No.:1432057-74-3
- 10-Acetoxyscandine
Catalog No.:BCN7035
CAS No.:1432058-90-6
- 3-(3-Hydroxy-3-methylbutanyl)-2,4,6-trihydroxybenzophenone
Catalog No.:BCC8588
CAS No.:1432062-53-7
- 8-Isomulberrin hydrate
Catalog No.:BCC8789
CAS No.:1432063-35-8
- 6-O-Acetylcoriatin
Catalog No.:BCN7048
CAS No.:1432063-63-2
- 10-O-Acetylisocalamendiol
Catalog No.:BCN7071
CAS No.:1432064-69-1
- (Z)-3-Hydroxy-5-methoxystilbene
Catalog No.:BCN6688
CAS No.:143207-76-5
Absorption, distribution, metabolism, and excretion of the novel antibacterial prodrug tedizolid phosphate.[Pubmed:24875463]
Drug Metab Dispos. 2014 Aug;42(8):1275-84.
Tedizolid phosphate is a novel antibacterial prodrug with potent activity against Gram-positive pathogens. In vitro and in vivo studies demonstrated that the prodrug is rapidly converted by nonspecific phosphatases to the biologically active moiety tedizolid. Single oral dose radiolabeled (14)C-tedizolid phosphate kinetic studies in human subjects (100 microCi in 204 mg tedizolid phosphate free acid) confirmed a rapid time to maximum tedizolid concentration (Tmax, 1.28 hours), a long terminal half-life (10.6 hours), and a Cmax of 1.99 microg/ml. Metabolite analysis of plasma, fecal, and urine samples from rats, dogs, and humans confirmed that tedizolid is the only measurable metabolite in plasma after intravenous (in animals only) or oral administration and that tedizolid sulfate is the major metabolite excreted from the body. Excellent mass balance recovery was achieved and demonstrated that fecal excretion is the predominant (80-90%) route of elimination across species, primarily as tedizolid sulfate. Urine excretion accounted for the balance of drug elimination but contained a broader range of minor metabolites. Glucuronidation products were not detected. Similar results were observed in rats and dogs after both intravenous and oral administration. The tedizolid metabolites showed less potent antibacterial activity than tedizolid. The observations from these studies support once daily dosing of tedizolid phosphate and highlight important metabolism and excretion features that differentiate tedizolid phosphate from linezolid.


