BMX-IN-1BMX (also termed ETK) kinase inhibitor CAS# 1431525-23-3 |
- LY2835219
Catalog No.:BCC1113
CAS No.:1231930-82-7
- Roscovitine (Seliciclib,CYC202)
Catalog No.:BCC1105
CAS No.:186692-46-6
- Nu 6027
Catalog No.:BCC1154
CAS No.:220036-08-8
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- AT7519 Hydrochloride
Catalog No.:BCC1376
CAS No.:902135-91-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1431525-23-3 | SDF | Download SDF |
| PubChem ID | 71576693 | Appearance | Powder |
| Formula | C29H24N4O4S | M.Wt | 524.59 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BMX kinase inhibitor | ||
| Solubility | DMSO : 100 mg/mL (190.63 mM; Need ultrasonic) DMF : 10 mg/mL (19.06 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
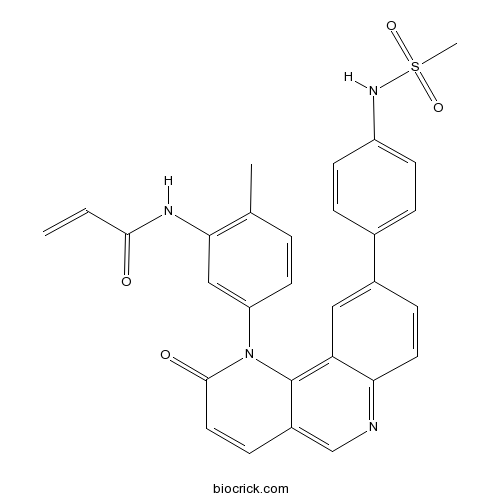
| Chemical Name | N-[5-[9-[4-(methanesulfonamido)phenyl]-2-oxobenzo[h][1,6]naphthyridin-1-yl]-2-methylphenyl]prop-2-enamide | ||
| SMILES | CC1=C(C=C(C=C1)N2C(=O)C=CC3=CN=C4C=CC(=CC4=C32)C5=CC=C(C=C5)NS(=O)(=O)C)NC(=O)C=C | ||
| Standard InChIKey | SFMJNHNUOVADRW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C29H24N4O4S/c1-4-27(34)31-26-16-23(12-5-18(26)2)33-28(35)14-9-21-17-30-25-13-8-20(15-24(25)29(21)33)19-6-10-22(11-7-19)32-38(3,36)37/h4-17,32H,1H2,2-3H3,(H,31,34) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | BMX-IN-1 is a highly selective and potent irreversible inhibitor of BMX kinase. | |||||
| Targets | BMX Kinase | |||||

BMX-IN-1 Dilution Calculator

BMX-IN-1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9063 mL | 9.5313 mL | 19.0625 mL | 38.125 mL | 47.6563 mL |
| 5 mM | 0.3813 mL | 1.9063 mL | 3.8125 mL | 7.625 mL | 9.5313 mL |
| 10 mM | 0.1906 mL | 0.9531 mL | 1.9063 mL | 3.8125 mL | 4.7656 mL |
| 50 mM | 0.0381 mL | 0.1906 mL | 0.3813 mL | 0.7625 mL | 0.9531 mL |
| 100 mM | 0.0191 mL | 0.0953 mL | 0.1906 mL | 0.3813 mL | 0.4766 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BMX-IN-1 is a highly selective and irreversible inhibitor of BMX kinase [1].
BMX, also known as ETK, is a member of Tec family and plays an important role in regulating ischemia-mediated arteriogenesis and lymphangiogenesis. BMX is mainly expressed in arterial endothelia and in myeloid hematopoietic cells and it has been reported that BMX involves in tumor growth [2].
BMX-IN-1 is a potent BMX kinase inhibitor and has an irreversible function on BMX kinase. When tested with prostate cancer cell lines, BMX-IN-1 treatment inhibited the proliferation of Tel-BMX-transformed Ba/F3 cells at lowdose [1].
References:
[1].Liu, F., et al., Discovery of a selective irreversible BMX inhibitor for prostate cancer. ACS Chem Biol, 2013. 8(7): p. 1423-8.
[2].Holopainen, T., et al., Deletion of the endothelial Bmx tyrosine kinase decreases tumor angiogenesis and growth. Cancer Res, 2012. 72(14): p. 3512-21.
- GSK-LSD1 2HCl
Catalog No.:BCC5647
CAS No.:1431368-48-7
- Neotuberostemonine
Catalog No.:BCN6239
CAS No.:143120-46-1
- BQ-3020
Catalog No.:BCC5728
CAS No.:143113-45-5
- Phytic acid sodium salt hydrate
Catalog No.:BCN1283
CAS No.:14306-25-3
- Ethyl 3,4-dicaffeoylquinate
Catalog No.:BCN8004
CAS No.:143051-73-4
- 1alpha-Hydroxy VD4
Catalog No.:BCC1300
CAS No.:143032-85-3
- ML216
Catalog No.:BCC8061
CAS No.:1430213-30-1
- Vinblastine Sulfate
Catalog No.:BCN2292
CAS No.:143-67-9
- Protoveratrine A
Catalog No.:BCN5346
CAS No.:143-57-7
- Lauric acid
Catalog No.:BCN2635
CAS No.:143-07-7
- Salvianolic acid D
Catalog No.:BCN2369
CAS No.:142998-47-8
- Salvianolic acid E
Catalog No.:BCN8194
CAS No.:142998-46-7
- H-D-Ala-OMe.HCl
Catalog No.:BCC3199
CAS No.:14316-06-4
- UNC1999
Catalog No.:BCC4552
CAS No.:1431612-23-5
- CAL-130
Catalog No.:BCC1440
CAS No.:1431697-74-3
- CAL-130 Hydrochloride
Catalog No.:BCC1441
CAS No.:1431697-78-7
- gamma-secretase modulator 3
Catalog No.:BCC1585
CAS No.:1431697-84-5
- AT7519 trifluoroacetate
Catalog No.:BCC1377
CAS No.:1431697-85-6
- AT7867 dihydrochloride
Catalog No.:BCC1378
CAS No.:1431697-86-7
- OTSSP167
Catalog No.:BCC4314
CAS No.:1431697-89-0
- SB-408124 Hydrochloride
Catalog No.:BCC1929
CAS No.:1431697-90-3
- CCT241533 hydrochloride
Catalog No.:BCC1463
CAS No.:1431697-96-9
- KN-92 hydrochloride
Catalog No.:BCC1681
CAS No.:1431698-47-3
- (S)-Tedizolid
Catalog No.:BCC1294
CAS No.:1431699-67-0
[Role of bone marrow tyrosine kinase on chromosome X in the production of pro-inflammatory cytokines from mouse mononuclear-macrophages RAW264.7 induced by endotoxin/lipopolysaccharide and its mechanism].[Pubmed:28427134]
Zhonghua Shao Shang Za Zhi. 2017 Apr 20;33(4):211-216.
Objective: To investigate the role of bone marrow tyrosine kinase on chromosome X (BMX) in the production of tumor necrosis factor alpha (TNF-alpha) and interleukin-1beta (IL-1beta) from mouse mononuclear-macrophages induced by endotoxin/lipopolysaccharide (LPS) and its related mechanism. Methods: Mouse mononuclear-macrophages RAW264.7 were inoculated in 6-well plates and routinely cultured for the following experiments. (1) Cells were collected and divided into blank control group, LPS control group, and 75, 750, 7 500, 75 000 nmol/L BMX-IN-1 pretreatment groups according to the random number table, with 8 wells in each group. Cells in blank control group were routinely cultured for 25 h. Cells in LPS control group were routinely cultured for 24 h and stimulated by LPS in the final mass concentration (the same below) of 0.1 mug/mL for 1 h. Cells in the latter 4 groups were pretreated with BMX-IN-1 in the final molarity (the same below) of 75, 750, 7 500, 75 000 nmol/L for 24 h and stimulated by 0.1 mug/mL LPS for 1 h. The mRNA expression of TNF-alpha of cells in each group was determined by real-time fluorescent quantitative reverse transcription polymerase chain reaction (RT-PCR) to screen the optimum concentration of BMX-IN-1. (2) Cells were collected and divided into LPS control group and 2, 4, 8, 12, 18 h BMX-IN-1 pretreatment groups according to the random number table, with 8 wells in each group. Cells in LPS control group were stimulated by 0.1 mug/mL LPS for 1 h. Cells in the latter 5 groups were pretreated with optimum concentration of BMX-IN-1 for 2, 4, 8, 12, 18 h respectively and stimulated by 0.1 mug/mL LPS for 1 h. The mRNA expression of TNF-alpha of cells in each group was determined by real-time fluorescent quantitative RT-PCR to screen the optimum time for BMX-IN-1 pre-treatment. (3) Cells were collected and divided into blank control group, BMX-IN-1 control group, LPS control group, and BMX-IN-1+ LPS group according to the random number table, with 16 wells in each group. Cells in blank control group were routinely cultured for the optimum time plus 1 h. Cells in BMX-IN-1 control group were pretreated with BMX-IN-1 in the optimum concentration for the optimum time and then routinely cultured for 1 h. Cells in LPS control group were routinely cultured for the optimum time and then stimulated by 0.1 mug/mL LPS for 1 h. Cells in BMX-IN-1+ LPS group were pretreated with BMX-IN-1 in the optimum concentration for the optimum time and then stimulated by 0.1 mug/mL LPS for 1 h. The mRNA expressions of TNF-alpha and IL-1beta were determined by real-time fluorescent quantitative RT-PCR, and the activity of BMX and p38 mitogen-activated protein kinase (MAPK) were determined by Western blotting, with 8 samples in each determination. Data were processed with one-way analysis of variance and LSD test. Results: (1) Compared with that in blank control group, the mRNA expression of TNF-alpha of cells was significantly increased in the other 5 groups (with P values below 0.01). Compared with that in LPS control group, the mRNA expression of TNF-alpha of cells was decreased in each BMX-IN-1 pretreatment group, but only the mRNA expression of TNF-alpha of cells in 75 000 nmol/L BMX-IN-1 pretreatment group was significantly decreased (P<0.05). The optimum concentration of BMX-IN-1 was 75 000 nmol/L. (2) Compared with that in LPS control group, the mRNA expression of TNF-alpha of cells was not significantly changed in 2 and 4 h BMX-IN-1 pretreatment groups (with P values above 0.05) but significantly decreased in 8, 12, and 18 h BMX-IN-1 pretreatment groups (P<0.05 or P<0.01). The mRNA expression of TNF-alpha of cells in 12 h BMX-IN-1 pretreatment group was the lowest. The optimum time for BMX-IN-1 pre-treatment was 12 h. (3) The mRNA expressions of TNF-alpha and IL-1beta of cells in BMX-IN-1 control group were 0.97+/-0.13 and 0.98+/-0.06, respectively, which were similar to 1.00 of blank control group (with P values above 0.05). The mRNA expressions of TNF-alpha and IL-1beta of cells in LPS control group were 2.97+/-0.17 and 3.07+/-0.60, respectively, while those in BMX-IN-1+ LPS group were 2.31+/-0.94 and 2.55+/-0.73, respectively, with the 4 values significantly higher than those in blank control group (with P values below 0.01). The mRNA expressions of TNF-alpha and IL-1beta of cells in BMX-IN-1+ LPS group were significantly lower than those in LPS control group (with P values below 0.05). The activity values of BMX and p38MAPK of cells in BMX-IN-1 control group were 0.95+/-0.19 and 0.98+/-0.18, respectively, which were close to 1.00+/-0.14 and 1.00+/-0.22 of blank control group (with P values above 0.05). The activity values of BMX and p38MAPK of cells in LPS control group were 1.98+/-0.33 and 2.05+/-0.34, respectively, which were significantly higher than those of blank control group (with P values below 0.01). The activity values of BMX and p38MAPK of cells in BMX-IN-1+ LPS group were 1.00+/-0.17 and 1.67+/-0.27, respectively, which were obviously lower than those of LPS control group (P<0.05 or P<0.01). Conclusions: BMX can increase the production of pro-inflammatory cytokines TNF-alpha and IL-1beta from mouse mononuclear-macrophages induced by LPS, which may be associated with the activation of the p38MAPK pathway by BMX.
BMX/Etk promotes cell proliferation and tumorigenicity of cervical cancer cells through PI3K/AKT/mTOR and STAT3 pathways.[Pubmed:28514765]
Oncotarget. 2017 Jul 25;8(30):49238-49252.
Bone marrow X-linked kinase (BMX, also known as Etk) has been reported to be involved in cell proliferation, differentiation, apoptosis, migration and invasion in several types of tumors, but its role in cervical carcinoma remains poorly understood. In this study, we showed that BMX expression exhibits a gradually increasing trend from normal cervical tissue to cervical cancer in situ and then to invasive cervical cancer tissue. Through BMX-IN-1, a potent and irreversible BMX kinase inhibitor, inhibited the expression of BMX, the cell proliferation was significantly decreased. Knockdown of BMX in HeLa and SiHa cervical cancer cell lines using two different silencing technologies, TALEN and shRNA, inhibited cell growth in vitro and suppressed xenograft tumor formation in vivo, whereas overexpression of BMX in the cell line C-33A significantly increased cell proliferation. Furthermore, a mechanism study showed that silencing BMX blocked cell cycle transit from G0/G1 to S or G2/M phase, and knockdown of BMX inhibited the expression of p-AKT and p-STAT3. These results suggested that BMX can promote cell proliferation through PI3K/AKT/mTOR and STAT3 signaling pathways in cervical cancer cells.
Discovery of a selective irreversible BMX inhibitor for prostate cancer.[Pubmed:23594111]
ACS Chem Biol. 2013 Jul 19;8(7):1423-8.
BMX is a member of the TEC family of nonreceptor tyrosine kinases. We have used structure-based drug design in conjunction with kinome profiling to develop a potent, selective, and irreversible BMX kinase inhibitor, BMX-IN-1, which covalently modifies Cys496. BMX-IN-1 inhibits the proliferation of Tel-BMX-transformed Ba/F3 cells at two digit nanomolar concentrations but requires single digit micromolar concentrations to inhibit the proliferation of prostate cancer cell lines. Using a combinatorial kinase inhibitor screening strategy, we discovered that the allosteric Akt inhibitor, MK2206, is able to potentiate BMX inhibitor's antiproliferation efficacy against prostate cancer cells.
Structure-activity relationship investigation for benzonaphthyridinone derivatives as novel potent Bruton's tyrosine kinase (BTK) irreversible inhibitors.[Pubmed:28628824]
Eur J Med Chem. 2017 Sep 8;137:545-557.
Through a structure-based drug design approach, a tricyclic benzonaphthyridinone pharmacophore was used as a starting point for carrying out detailed medicinal structure-activity relationhip (SAR) studies geared toward characterization of a panel of proposed BTK inhibitors, including 6 (QL-X-138), 7 (BMX-IN-1) and 8 (QL47). These studies led to the discovery of the novel potent irreversible BTK inhibitor, compound 18 (CHMFL-BTK-11). Kinetic analysis of compound 18 revealed an irreversible binding efficacy (kinact/Ki) of 0.01 muM(-1)s(-1). Compound 18 potently inhibited BTK kinase Y223 auto-phosphorylation (EC50 < 100 nM), arrested cell cycle in G0/G1 phase, and induced apoptosis in Ramos, MOLM13 and Pfeiffer cells. We believe these features would make 18 a good pharmacological tool for studying BTK-related pathologies.


