GSK-LSD1 2HClCAS# 1431368-48-7 |
- Lenalidomide hydrochloride
Catalog No.:BCC1697
CAS No.:1243329-97-6
- GSK J1
Catalog No.:BCC2231
CAS No.:1373422-53-7
- Necrostatin-1
Catalog No.:BCC2247
CAS No.:4311-88-0
- IOX2(Glycine)
Catalog No.:BCC2229
CAS No.:931398-72-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1431368-48-7 | SDF | Download SDF |
| PubChem ID | 91663353 | Appearance | Powder |
| Formula | C14H22Cl2N2 | M.Wt | 289.24 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >11.55mg/mL in DMSO | ||
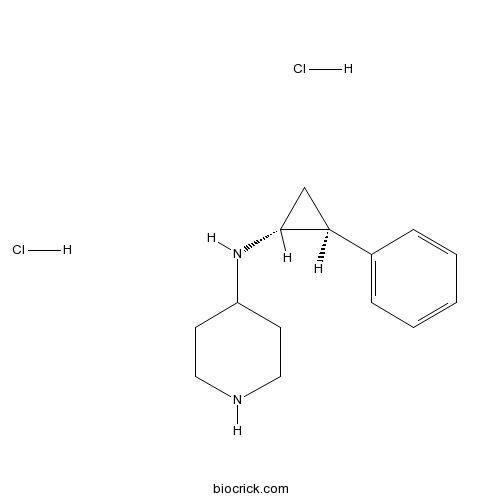
| Chemical Name | N-[(1R,2S)-2-phenylcyclopropyl]piperidin-4-amine;dihydrochloride | ||
| SMILES | C1CNCCC1NC2CC2C3=CC=CC=C3.Cl.Cl | ||
| Standard InChIKey | PJFZOGMSPBHPNS-WICJZZOFSA-N | ||
| Standard InChI | InChI=1S/C14H20N2.2ClH/c1-2-4-11(5-3-1)13-10-14(13)16-12-6-8-15-9-7-12;;/h1-5,12-16H,6-10H2;2*1H/t13-,14+;;/m0../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

GSK-LSD1 2HCl Dilution Calculator

GSK-LSD1 2HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4573 mL | 17.2867 mL | 34.5734 mL | 69.1467 mL | 86.4334 mL |
| 5 mM | 0.6915 mL | 3.4573 mL | 6.9147 mL | 13.8293 mL | 17.2867 mL |
| 10 mM | 0.3457 mL | 1.7287 mL | 3.4573 mL | 6.9147 mL | 8.6433 mL |
| 50 mM | 0.0691 mL | 0.3457 mL | 0.6915 mL | 1.3829 mL | 1.7287 mL |
| 100 mM | 0.0346 mL | 0.1729 mL | 0.3457 mL | 0.6915 mL | 0.8643 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GSK-LSD1 2HCl is an irreversible, and selective LSD1 inhibitor with IC50 of 16 nM, > 1000 fold selective over other closely related FAD utilizing enzymes (i.e. LSD2, MAO-A, MAO-B).
- Neotuberostemonine
Catalog No.:BCN6239
CAS No.:143120-46-1
- BQ-3020
Catalog No.:BCC5728
CAS No.:143113-45-5
- Phytic acid sodium salt hydrate
Catalog No.:BCN1283
CAS No.:14306-25-3
- Ethyl 3,4-dicaffeoylquinate
Catalog No.:BCN8004
CAS No.:143051-73-4
- 1alpha-Hydroxy VD4
Catalog No.:BCC1300
CAS No.:143032-85-3
- ML216
Catalog No.:BCC8061
CAS No.:1430213-30-1
- Vinblastine Sulfate
Catalog No.:BCN2292
CAS No.:143-67-9
- Protoveratrine A
Catalog No.:BCN5346
CAS No.:143-57-7
- Lauric acid
Catalog No.:BCN2635
CAS No.:143-07-7
- Salvianolic acid D
Catalog No.:BCN2369
CAS No.:142998-47-8
- Salvianolic acid E
Catalog No.:BCN8194
CAS No.:142998-46-7
- Fmoc-D-Ala(3-pyridyl)-OH.HCl
Catalog No.:BCC3324
CAS No.:142994-45-4
- BMX-IN-1
Catalog No.:BCC1434
CAS No.:1431525-23-3
- H-D-Ala-OMe.HCl
Catalog No.:BCC3199
CAS No.:14316-06-4
- UNC1999
Catalog No.:BCC4552
CAS No.:1431612-23-5
- CAL-130
Catalog No.:BCC1440
CAS No.:1431697-74-3
- CAL-130 Hydrochloride
Catalog No.:BCC1441
CAS No.:1431697-78-7
- gamma-secretase modulator 3
Catalog No.:BCC1585
CAS No.:1431697-84-5
- AT7519 trifluoroacetate
Catalog No.:BCC1377
CAS No.:1431697-85-6
- AT7867 dihydrochloride
Catalog No.:BCC1378
CAS No.:1431697-86-7
- OTSSP167
Catalog No.:BCC4314
CAS No.:1431697-89-0
- SB-408124 Hydrochloride
Catalog No.:BCC1929
CAS No.:1431697-90-3
- CCT241533 hydrochloride
Catalog No.:BCC1463
CAS No.:1431697-96-9
- KN-92 hydrochloride
Catalog No.:BCC1681
CAS No.:1431698-47-3
The Smac mimetic RMT5265.2HCL induces apoptosis in EBV and HTLV-I associated lymphoma cells by inhibiting XIAP and promoting the mitochondrial release of cytochrome C and Smac.[Pubmed:22325366]
Leuk Res. 2012 Jun;36(6):784-90.
The inhibitors of apoptosis (IAP) are important regulators of apoptosis. However, little is known about the capacity of Smac mimetics (IAP inhibitor) to overcome virally associated-lymphoma's (VAL) resistance to apoptosis. Here, we explored the pro-apoptotic effect of a novel Smac mimetic, RMT5265.2HCL (RMT) in VAL cells. RMT improved the sensitivity to apoptosis in EBV- and to some extend in HTLV-1- but not in HHV-8-VAL. Furthermore, we identified that RMT promotes caspase 3 and 9 cleavage by inhibiting XIAP and inducing the mitochondrial efflux of Smac and cytochrome C. This investigation further support exploring the use of Smac inhibitors in VAL.
Probing the micellar properties of Quinacrine 2HCl and its binding with surfactants and human serum albumin.[Pubmed:23727671]
Spectrochim Acta A Mol Biomol Spectrosc. 2013 Sep;113:182-90.
This manuscript reports physicochemical behavior of an antimalarial drug Quinacrine 2HCl (QUN) drug as well as its interaction with surfactant and Human Serum Albumin (HSA). Surface tension and specific conductivity were employed to detect the critical micelle concentration (CMC) and thus its surface and thermodynamic parameters were calculated. Solublization of this drug within micelles of anionic surfactant sodium dodecyl sulfate (SDS) has also been studied. UV/Visible spectroscopy was used to calculate partition coefficient (Kx), free energy of partition and number of drug molecules per micelle. The complexation of drug with HSA at physiological conditions (pH 7.4) has been analyzed by using UV/Visible and fluorescence spectroscopy. In this way the values of drug-protein binding constant, number of binding sites and free energy of binding were calculated.
Reduction of oxidative stress in adjuvant arthritis. Comparison of efficacy of two pyridoindoles: stobadine dipalmitate and SMe1.2HCl.[Pubmed:20548970]
Acta Biochim Pol. 2010;57(2):223-8. Epub 2010 Jun 14.
The aim of this study was to evaluate the therapeutic potential of oxidative stress (OS) reduction by using pyridoindole (PI) antioxidants in adjuvant arthritis (AA). The substances tested were stobadine dipalmitate (STB) and SMe1. AA was used as animal model. The experiments included healthy animals, control arthritic animals and arthritic animals with administration of PI in the oral daily dose of 15 mg/kg b.m during 28 experimental days. The rats were sacrificed on day 28. Clinical and biochemical parameters were determined. The effect of PI administration was evaluated on the basis of the following parameters: (a) arthritis (volume of hind paws - HPW, change of animal body mass - CBM), (b) OS (chemiluminescence of whole blood - CWB, levels of thiobarbituric acid reacting substance - TBARS and of HNE- and MDA-protein adducts in plasma and activity of gamma-glutamyltransferase (GGT) in hind paw joint homogenates). The PI studied significantly increased the CBM of animals and corrected the HPW. STB also significantly decreased the activity of GGT in joint homogenates. SMe1 was more effective in decreasing plasmatic TBARS levels, but STB was more effective in reducing plasmatic HNE- and MDA-protein adducts. The assay for HNE- and MDA-adducts in plasma as a function of time was applied for the first time in AA. STB markedly decreased spontaneous and PMA-stimulated CWB and reduced neutrophil count. In summary, STB was more effective than SMe1 in reducing OS in AA. Our results showed that the reduction of OS in arthritis also corrected the clinical manifestations of the disease.


