ML216BLM helicase inhibitor CAS# 1430213-30-1 |
- THZ1
Catalog No.:BCC4005
CAS No.:1604810-83-4
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- AZD-5438
Catalog No.:BCC3689
CAS No.:602306-29-6
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- PD 0332991 (Palbociclib) HCl
Catalog No.:BCC3680
CAS No.:827022-32-2
- Palbociclib (PD0332991) Isethionate
Catalog No.:BCC3698
CAS No.:827022-33-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1430213-30-1 | SDF | Download SDF |
| PubChem ID | 49852229 | Appearance | Powder |
| Formula | C15H9F4N5OS | M.Wt | 383.32 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 20 mg/mL (52.18 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
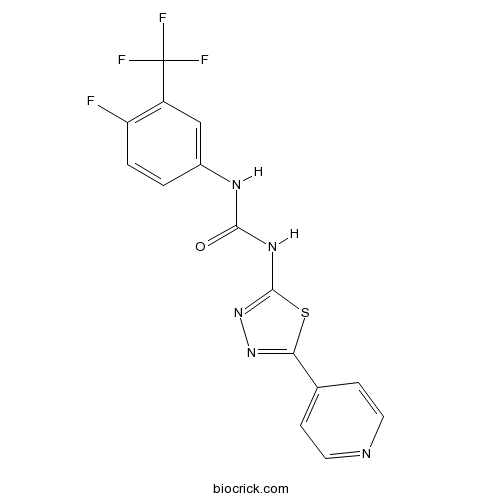
| Chemical Name | 1-[4-fluoro-3-(trifluoromethyl)phenyl]-3-(5-pyridin-4-yl-1,3,4-thiadiazol-2-yl)urea | ||
| SMILES | C1=CC(=C(C=C1NC(=O)NC2=NN=C(S2)C3=CC=NC=C3)C(F)(F)F)F | ||
| Standard InChIKey | WMCOYUSJXXCHFH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H9F4N5OS/c16-11-2-1-9(7-10(11)15(17,18)19)21-13(25)22-14-24-23-12(26-14)8-3-5-20-6-4-8/h1-7H,(H2,21,22,24,25) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

ML216 Dilution Calculator

ML216 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6088 mL | 13.0439 mL | 26.0879 mL | 52.1757 mL | 65.2197 mL |
| 5 mM | 0.5218 mL | 2.6088 mL | 5.2176 mL | 10.4351 mL | 13.0439 mL |
| 10 mM | 0.2609 mL | 1.3044 mL | 2.6088 mL | 5.2176 mL | 6.522 mL |
| 50 mM | 0.0522 mL | 0.2609 mL | 0.5218 mL | 1.0435 mL | 1.3044 mL |
| 100 mM | 0.0261 mL | 0.1304 mL | 0.2609 mL | 0.5218 mL | 0.6522 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: 3.0 and 0.97 μM for full length BLM and BLM636–1298, respectively
ML216 is a potent inhibitor of the DNA unwinding activity of BLM helicase.
BLM helicase is reported to be a DNA unwinding enzyme critical in DNA repair through the homologous recombination pathway. BLM gene mutations lead to diminished BLM helicase activity and can cause Bloom’s Syndrome. Similar to other DNA repair enzymes, BLM helicase inhibition shows sensitization of tumor cells to conventional cancer therapies, such as camptothecin.
In vitro: ML216 showed submicromolar potency and selectivity over related helicases including RECQ1, RECQ5, and E. coli UvrD helicases. ML216 also inhibited cell proliferation of BLM-proficient fibroblast cells while had minimal effects on BLM deficient fibroblast cells, indicating on-target activity in a cellular context. Additionally, ML216 increased the frequency of sister chromatid exchanges, which was a diagnostic cellular phenotype consistent with the absence of a functional BLM protein [1].
In vivo: ML216 was a suitable starting point for further mouse tumor xenograft models and for the further development of potential cancer therapeutics [1].
Clinical trial: N/A
Reference:
[1] Rosenthal AS,Dexheimer TS,Nguyen G,Gileadi O,Vindigni A,Simeonov A,Jadhav A,Hickson I,Maloney DJ. Discovery of ML216, a Small Molecule Inhibitor of Bloom (BLM) Helicase. Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010-2011 Apr 15
- Vinblastine Sulfate
Catalog No.:BCN2292
CAS No.:143-67-9
- Protoveratrine A
Catalog No.:BCN5346
CAS No.:143-57-7
- Lauric acid
Catalog No.:BCN2635
CAS No.:143-07-7
- Salvianolic acid D
Catalog No.:BCN2369
CAS No.:142998-47-8
- Salvianolic acid E
Catalog No.:BCN8194
CAS No.:142998-46-7
- Fmoc-D-Ala(3-pyridyl)-OH.HCl
Catalog No.:BCC3324
CAS No.:142994-45-4
- Fmoc-D-Phe(4-Cl)-OH
Catalog No.:BCC3177
CAS No.:142994-19-2
- UNC2025
Catalog No.:BCC8062
CAS No.:1429881-91-3
- HPOB
Catalog No.:BCC5574
CAS No.:1429651-50-2
- Triptoquinone A
Catalog No.:BCN6781
CAS No.:142950-86-5
- Triptoquinone B
Catalog No.:BCN6238
CAS No.:142937-50-6
- Mutant IDH1 inhibitor
Catalog No.:BCC4144
CAS No.:1429180-08-4
- 1alpha-Hydroxy VD4
Catalog No.:BCC1300
CAS No.:143032-85-3
- Ethyl 3,4-dicaffeoylquinate
Catalog No.:BCN8004
CAS No.:143051-73-4
- Phytic acid sodium salt hydrate
Catalog No.:BCN1283
CAS No.:14306-25-3
- BQ-3020
Catalog No.:BCC5728
CAS No.:143113-45-5
- Neotuberostemonine
Catalog No.:BCN6239
CAS No.:143120-46-1
- GSK-LSD1 2HCl
Catalog No.:BCC5647
CAS No.:1431368-48-7
- BMX-IN-1
Catalog No.:BCC1434
CAS No.:1431525-23-3
- H-D-Ala-OMe.HCl
Catalog No.:BCC3199
CAS No.:14316-06-4
- UNC1999
Catalog No.:BCC4552
CAS No.:1431612-23-5
- CAL-130
Catalog No.:BCC1440
CAS No.:1431697-74-3
- CAL-130 Hydrochloride
Catalog No.:BCC1441
CAS No.:1431697-78-7
- gamma-secretase modulator 3
Catalog No.:BCC1585
CAS No.:1431697-84-5
Genetic mapping of species differences via in vitro crosses in mouse embryonic stem cells.[Pubmed:29563231]
Proc Natl Acad Sci U S A. 2018 Apr 3;115(14):3680-3685.
Discovering the genetic changes underlying species differences is a central goal in evolutionary genetics. However, hybrid crosses between species in mammals often suffer from hybrid sterility, greatly complicating genetic mapping of trait variation across species. Here, we describe a simple, robust, and transgene-free technique to generate "in vitro crosses" in hybrid mouse embryonic stem (ES) cells by inducing random mitotic cross-overs with the drug ML216, which inhibits the DNA helicase Bloom syndrome (BLM). Starting with an interspecific F1 hybrid ES cell line between the Mus musculus laboratory mouse and Mus spretus ( approximately 1.5 million years of divergence), we mapped the genetic basis of drug resistance to the antimetabolite tioguanine to a single region containing hypoxanthine-guanine phosphoribosyltransferase (Hprt) in as few as 21 d through "flow mapping" by coupling in vitro crosses with fluorescence-activated cell sorting (FACS). We also show how our platform can enable direct study of developmental variation by rederiving embryos with contribution from the recombinant ES cell lines. We demonstrate how in vitro crosses can overcome major bottlenecks in mouse complex trait genetics and address fundamental questions in evolutionary biology that are otherwise intractable through traditional breeding due to high cost, small litter sizes, and/or hybrid sterility. In doing so, we describe an experimental platform toward studying evolutionary systems biology in mouse and potentially in human and other mammals, including cross-species hybrids.
Pacemaker role of pericytes in generating synchronized spontaneous Ca2+ transients in the myenteric microvasculature of the guinea-pig gastric antrum.[Pubmed:26153078]
Cell Calcium. 2015 Nov;58(5):442-56.
Properties of spontaneous Ca(2+) transients in the myenteric microvasculature of the guinea-pig stomach were investigated. Specifically, we explored the spatio-temporal origin of Ca(2+) transients and the role of voltage-dependent Ca(2+) channels (VDCCs) in their intercellular synchrony using fluorescence Ca(2+) imaging and immunohistochemistry. The microvasculature generated spontaneous Ca(2+) transients that were independent of both Ca(2+) transients in interstitial cells of Cajal (ICC) and neural activity. Spontaneous Ca(2+) transients were highly synchronous along the length of microvasculature, and appeared to be initiated in pericytes and spread to arteriolar smooth muscle cells (SMCs). In most cases, the generation or synchrony of Ca(2+) transients was not affected by blockers of L-type VDCCs. In nifedipine-treated preparations, synchronous spontaneous Ca(2+) transients were readily blocked by Ni(2+), mibefradil or ML216, blockers for T-type VDCCs. These blockers also suppressed the known T-type VDCC dependent component of ICC Ca(2+) transients or slow waves. Spontaneous Ca(2+) transients were also suppressed by caffeine, tetracaine or cyclopiazonic acid (CPA). After the blockade of both L- and T-type VDCCs, asynchronous Ca(2+) transients were generated in pericytes on precapillary arterioles and/or capillaries but not in arteriolar SMCs, and were abolished by CPA or nominally Ca(2+) free solution. Together these data indicate that pericytes in the myenteric microvasculature may act as the origin of synchronous spontaneous Ca(2+) transients. Pericyte Ca(2+) transients arise from Ca(2+) release from the sarco-endoplasmic reticulum and the opening of T-type Ca(2+) VDCCs is required for their synchrony and propagation to arteriolar SMCs.
Synthesis and SAR studies of 5-(pyridin-4-yl)-1,3,4-thiadiazol-2-amine derivatives as potent inhibitors of Bloom helicase.[Pubmed:24012121]
Bioorg Med Chem Lett. 2013 Oct 15;23(20):5660-6.
Human cells utilize a variety of complex DNA repair mechanisms in order to combat constant mutagenic and cytotoxic threats from both exogenous and endogenous sources. The RecQ family of DNA helicases, which includes Bloom helicase (BLM), plays an important function in DNA repair by unwinding complementary strands of duplex DNA as well as atypical DNA structures such as Holliday junctions. Mutations of the BLM gene can result in Bloom syndrome, an autosomal recessive disorder associated with cancer predisposition. BLM-deficient cells exhibit increased sensitivity to DNA damaging agents indicating that a selective BLM inhibitor could be useful in potentiating the anticancer activity of these agents. In this work, we describe the medicinal chemistry optimization of the hit molecule following a quantitative high-throughput screen of >355,000 compounds. These efforts lead to the identification of ML216 and related analogs, which possess potent BLM inhibition and exhibit selectivity over related helicases. Moreover, these compounds demonstrated cellular activity by inducing sister chromatid exchanges, a hallmark of Bloom syndrome.
A small molecule inhibitor of the BLM helicase modulates chromosome stability in human cells.[Pubmed:23352139]
Chem Biol. 2013 Jan 24;20(1):55-62.
The Bloom's syndrome protein, BLM, is a member of the conserved RecQ helicase family. Although cell lines lacking BLM exist, these exhibit progressive genomic instability that makes distinguishing primary from secondary effects of BLM loss problematic. In order to be able to acutely disable BLM function in cells, we undertook a high throughput screen of a chemical compound library for small molecule inhibitors of BLM. We present ML216, a potent inhibitor of the DNA unwinding activity of BLM. ML216 shows cell-based activity and can induce sister chromatid exchanges, enhance the toxicity of aphidicolin, and exert antiproliferative activity in cells expressing BLM, but not those lacking BLM. These data indicate that ML216 shows strong selectivity for BLM in cultured cells. We discuss the potential utility of such a BLM-targeting compound as an anticancer agent.


