UNC2025CAS# 1429881-91-3 |
- TCS 359
Catalog No.:BCC1183
CAS No.:301305-73-7
- Cabozantinib (XL184, BMS-907351)
Catalog No.:BCC1264
CAS No.:849217-68-1
- Amuvatinib (MP-470, HPK 56)
Catalog No.:BCC2258
CAS No.:850879-09-3
- TG101209
Catalog No.:BCC2198
CAS No.:936091-14-4
- Ponatinib (AP24534)
Catalog No.:BCC2522
CAS No.:943319-70-8
- Quizartinib (AC220)
Catalog No.:BCC2548
CAS No.:950769-58-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1429881-91-3 | SDF | Download SDF |
| PubChem ID | 73425588 | Appearance | Powder |
| Formula | C28H40N6O | M.Wt | 476.66 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >23.9mg/mL in DMSO | ||
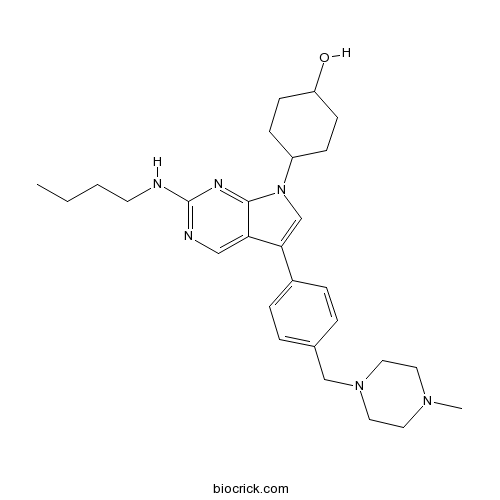
| Chemical Name | 4-[2-(butylamino)-5-[4-[(4-methylpiperazin-1-yl)methyl]phenyl]pyrrolo[2,3-d]pyrimidin-7-yl]cyclohexan-1-ol | ||
| SMILES | CCCCNC1=NC=C2C(=CN(C2=N1)C3CCC(CC3)O)C4=CC=C(C=C4)CN5CCN(CC5)C | ||
| Standard InChIKey | MJSHVHLADKXCML-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C28H40N6O/c1-3-4-13-29-28-30-18-25-26(20-34(27(25)31-28)23-9-11-24(35)12-10-23)22-7-5-21(6-8-22)19-33-16-14-32(2)15-17-33/h5-8,18,20,23-24,35H,3-4,9-17,19H2,1-2H3,(H,29,30,31) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | UNC2025 is a potent and orally bioavailable Mer/Flt3 dual inhibitor with IC50 of 0.8/0.74 nM for Mer/Flt3.
IC50 value: 0.8/0.74 nM(MER/FLT3)
Target: Mer/Flt3 inhibitor
UNC2025 was capable of inhibiting Mer phosphorylation in vivo, following oral dosing as demonstrated by pharmaco-dynamic (PD) studies examining phospho-Mer in leukemic blasts from mouse bone marrow. Kinome pro ling versus more than 300 kinases in vitro and cellular selectivity assessments demonstrate that 11 has similar subnanomolar activity against Flt3, an additional important target in acute myelogenous leukemia (AML), with pharmacologically useful selectivity versus other kinases examined. References: | |||||

UNC2025 Dilution Calculator

UNC2025 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0979 mL | 10.4897 mL | 20.9793 mL | 41.9586 mL | 52.4483 mL |
| 5 mM | 0.4196 mL | 2.0979 mL | 4.1959 mL | 8.3917 mL | 10.4897 mL |
| 10 mM | 0.2098 mL | 1.049 mL | 2.0979 mL | 4.1959 mL | 5.2448 mL |
| 50 mM | 0.042 mL | 0.2098 mL | 0.4196 mL | 0.8392 mL | 1.049 mL |
| 100 mM | 0.021 mL | 0.1049 mL | 0.2098 mL | 0.4196 mL | 0.5245 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
UNC2025 is a potent and orally bioavailable Mer/Flt3 dual inhibitor with IC50 of 0.8/0.74 nM for Mer/Flt3.
- HPOB
Catalog No.:BCC5574
CAS No.:1429651-50-2
- Triptoquinone A
Catalog No.:BCN6781
CAS No.:142950-86-5
- Triptoquinone B
Catalog No.:BCN6238
CAS No.:142937-50-6
- Mutant IDH1 inhibitor
Catalog No.:BCC4144
CAS No.:1429180-08-4
- OXF BD 02
Catalog No.:BCC5598
CAS No.:1429129-68-9
- Petunidin chloride
Catalog No.:BCN3018
CAS No.:1429-30-7
- 8alpha-Methacryloyloxy-13-ethoxyvernojalcanolide
Catalog No.:BCN7445
CAS No.:142891-14-3
- 1-O-Ethylpiptocarphin F
Catalog No.:BCN6448
CAS No.:142891-12-1
- GM 6001
Catalog No.:BCC2119
CAS No.:142880-36-2
- U 73343
Catalog No.:BCC8091
CAS No.:142878-12-4
- Toddacoumaquinone
Catalog No.:BCN3640
CAS No.:142878-03-3
- N-Acetyl-N-acetoxy-4-chlorobenzenesulfonamide
Catalog No.:BCC6762
CAS No.:142867-52-5
- Fmoc-D-Phe(4-Cl)-OH
Catalog No.:BCC3177
CAS No.:142994-19-2
- Fmoc-D-Ala(3-pyridyl)-OH.HCl
Catalog No.:BCC3324
CAS No.:142994-45-4
- Salvianolic acid E
Catalog No.:BCN8194
CAS No.:142998-46-7
- Salvianolic acid D
Catalog No.:BCN2369
CAS No.:142998-47-8
- Lauric acid
Catalog No.:BCN2635
CAS No.:143-07-7
- Protoveratrine A
Catalog No.:BCN5346
CAS No.:143-57-7
- Vinblastine Sulfate
Catalog No.:BCN2292
CAS No.:143-67-9
- ML216
Catalog No.:BCC8061
CAS No.:1430213-30-1
- 1alpha-Hydroxy VD4
Catalog No.:BCC1300
CAS No.:143032-85-3
- Ethyl 3,4-dicaffeoylquinate
Catalog No.:BCN8004
CAS No.:143051-73-4
- Phytic acid sodium salt hydrate
Catalog No.:BCN1283
CAS No.:14306-25-3
- BQ-3020
Catalog No.:BCC5728
CAS No.:143113-45-5
UNC2025, a potent and orally bioavailable MER/FLT3 dual inhibitor.[Pubmed:25068800]
J Med Chem. 2014 Aug 28;57(16):7031-41.
We previously reported a potent small molecule Mer tyrosine kinase inhibitor UNC1062. However, its poor PK properties prevented further assessment in vivo. We report here the sequential modification of UNC1062 to address DMPK properties and yield a new potent and highly orally bioavailable Mer inhibitor, 11, capable of inhibiting Mer phosphorylation in vivo, following oral dosing as demonstrated by pharmaco-dynamic (PD) studies examining phospho-Mer in leukemic blasts from mouse bone marrow. Kinome profiling versus more than 300 kinases in vitro and cellular selectivity assessments demonstrate that 11 has similar subnanomolar activity against Flt3, an additional important target in acute myelogenous leukemia (AML), with pharmacologically useful selectivity versus other kinases examined.
UNC2025, a MERTK Small-Molecule Inhibitor, Is Therapeutically Effective Alone and in Combination with Methotrexate in Leukemia Models.[Pubmed:27649555]
Clin Cancer Res. 2017 Mar 15;23(6):1481-1492.
Purpose: MERTK tyrosine kinase is ectopically expressed in 30% to 50% of acute lymphoblastic leukemias (ALL) and more than 80% of acute myeloid leukemias (AML) and is a potential therapeutic target. Here, we evaluated the utility of UNC2025, a MERTK tyrosine kinase inhibitor, for treatment of acute leukemia.Experimental Design: Preclinical in vitro and in vivo assays using cell lines and primary leukemia patient samples were used to evaluate antileukemic effects of UNC2025.Results: UNC2025 potently inhibited prosurvival signaling, induced apoptosis, and reduced proliferation and colony formation in MERTK-expressing ALL and AML cell lines and patient samples. Approximately 30% of primary leukemia patient samples (78 of 261 total) were sensitive to UNC2025. Sensitive samples were most prevalent in the AML, T-ALL, and minimally differentiated (M0) AML subsets. UNC2025 inhibited MERTK in bone marrow leukemia cells and had significant therapeutic effects in xenograft models, with dose-dependent decreases in tumor burden and consistent two-fold increases in median survival, irrespective of starting disease burden. In a patient-derived AML xenograft model, treatment with UNC2025 induced disease regression. In addition, UNC2025 increased sensitivity to methotrexate in vivo, suggesting that addition of MERTK-targeted therapy to current cytotoxic regimens may be particularly effective and/or allow for chemotherapy dose reduction.Conclusions: The broad-spectrum activity mediated by UNC2025 in leukemia patient samples and xenograft models, alone or in combination with cytotoxic chemotherapy, supports continued development of MERTK inhibitors for treatment of leukemia. Clin Cancer Res; 23(6); 1481-92. (c)2016 AACR.


