Quizartinib (AC220)FLT3 inhibitor,potent and selective CAS# 950769-58-1 |
- TCS 359
Catalog No.:BCC1183
CAS No.:301305-73-7
- Tandutinib (MLN518)
Catalog No.:BCC4499
CAS No.:387867-13-2
- Amuvatinib (MP-470, HPK 56)
Catalog No.:BCC2258
CAS No.:850879-09-3
- TG101209
Catalog No.:BCC2198
CAS No.:936091-14-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 950769-58-1 | SDF | Download SDF |
| PubChem ID | 24889392 | Appearance | Powder |
| Formula | C29H32N6O4S | M.Wt | 560.67 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AC220 | ||
| Solubility | DMSO : ≥ 33 mg/mL (58.86 mM) *"≥" means soluble, but saturation unknown. | ||
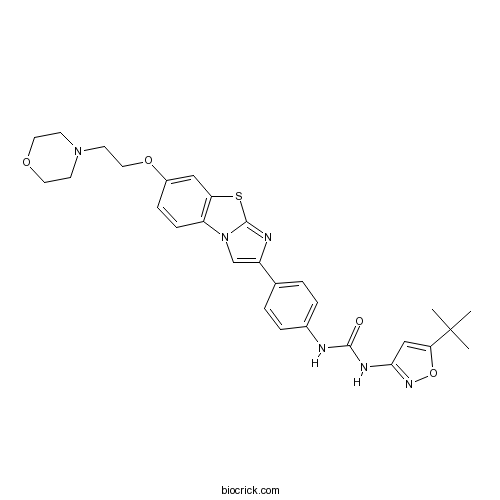
| Chemical Name | 1-(5-tert-butyl-1,2-oxazol-3-yl)-3-[4-[6-(2-morpholin-4-ylethoxy)imidazo[2,1-b][1,3]benzothiazol-2-yl]phenyl]urea | ||
| SMILES | CC(C)(C)C1=CC(=NO1)NC(=O)NC2=CC=C(C=C2)C3=CN4C5=C(C=C(C=C5)OCCN6CCOCC6)SC4=N3 | ||
| Standard InChIKey | CVWXJKQAOSCOAB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C29H32N6O4S/c1-29(2,3)25-17-26(33-39-25)32-27(36)30-20-6-4-19(5-7-20)22-18-35-23-9-8-21(16-24(23)40-28(35)31-22)38-15-12-34-10-13-37-14-11-34/h4-9,16-18H,10-15H2,1-3H3,(H2,30,32,33,36) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Quizartinib (AC220) is a second-generation inhibitor of FLT3 for Flt3(ITD/WT) with IC50 of 1.1 nM/4.2 nM, 10-fold more selective for Flt3 than KIT, PDGFRα, PDGFRβ, RET, and CSF-1R. | |||||
| Targets | Flt3ITD | Flt3wt | ||||
| IC50 | 1.1 nM | 4.2 nM | ||||

Quizartinib (AC220) Dilution Calculator

Quizartinib (AC220) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7836 mL | 8.9179 mL | 17.8358 mL | 35.6716 mL | 44.5895 mL |
| 5 mM | 0.3567 mL | 1.7836 mL | 3.5672 mL | 7.1343 mL | 8.9179 mL |
| 10 mM | 0.1784 mL | 0.8918 mL | 1.7836 mL | 3.5672 mL | 4.459 mL |
| 50 mM | 0.0357 mL | 0.1784 mL | 0.3567 mL | 0.7134 mL | 0.8918 mL |
| 100 mM | 0.0178 mL | 0.0892 mL | 0.1784 mL | 0.3567 mL | 0.4459 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Quizartinib (AC220) is a 2nd-generation FLT3 inhibitor for Flt3(ITD/WT) with IC50 value of 1.1 nM/4.2 nM, and it is ten-fold more selective for Flt3 than PDGFRα, PDGFRβ, KIT, RET and CSF-1R [1].
Quizartinib inhibits FLT3 with low nanomolar potency in cellular assays and shows high selectivity when screened against most of the human protein kinome. In addition, the combination of high potency and selectivity exhibited by quizartinib is unique compared with CEP-701, PKC-412, MLN-518, sunitinib, and sorafenib.
Quizartinib (AC220) was identified to be the most potent and selective FLT3 inhibitor with good pharmaceutical properties and superior efficacy in tumor xenograft models. A single dose of 10 mg/kg was administered to mice by oral gavage and plasma levels were measured over a 24-hour period. Quizartinib was well absorbed, achieving a maximum plasma level (Cmax) of 3.8 μM (2100 ng/mL) within 2 hours of dosing. To determine the effect of FLT3-ITD inhibition on cell growth,. These results establish that AC220 has strong activity against FLT3 in biochemical and cellular assays in MV4-11 cell proliferation in the presence of 1.1nM quizartinib [1].
As a FLT3 inhibitor for the treatment of acute myeloid leukemia (AML), when at doses as low as 1 mg/kg orally once a day, quizartinib inhibits FLT3 activity in vivo extending survival significantly. And this eradicates tumors in a FLT3-dependent mouse xenograft model, and potently inhibits FLT3 activity in primary patient cells at a dose of 10 mg/kg. In addition, quizartinib has been demonstrated a desirable safety and PK profile in humans. The emergence of resistant mutations is a common mechanism of resistance to FLT3 inhibitors used clinically, with a mutation emerging in at least 20% of the patients. This shows that the survival of AML blasts depends to a great extent on FLT3 signaling in these cases [2, 3].
References:
[1]. Zarrinkar PP, Gunawardane RN, Cramer MD, et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML). Blood, 2009, 114(14): 2984-2992.
[2]. Chao Q, Sprankle KG, Grotzfeld RM, et al. Identification of N-(5-tert-Butyl-isoxazol-3-yl)-N'-{4-[7-(2-morpholin-4-yl-ethoxy)imidazo[2,1-b][1,3]benzothiazol-2-yl]phenyl}urea Dihydrochloride (AC220), a Uniquely Potent, Selective, and Efficacious FMS-Like Tyrosine Kinase-3 (FLT3) Inhibitor. Journal of Medicinal Chemistry, 2009, 52(23): 7808-7816.
[3]. Alvarado Y , Kantarjian HM, Luthra R, et al. Treatment With FLT3 Inhibitor in Patients With FLT3-Mutated Acute Myeloid Leukemia Is Associated With Development of Secondary FLT3-Tyrosine Kinase Domain Mutations. Cancer, 2014, 120(14): 2142-2149.
- PCI-34051
Catalog No.:BCC2148
CAS No.:950762-95-5
- B-Raf IN 1
Catalog No.:BCC5439
CAS No.:950736-05-7
- 6-O-Methacryloyltrilobolide
Catalog No.:BCN7599
CAS No.:950685-51-5
- Gemcitabine
Catalog No.:BCC3784
CAS No.:95058-81-4
- Liproxstatin-1
Catalog No.:BCC5651
CAS No.:950455-15-9
- Erianin
Catalog No.:BCN2350
CAS No.:95041-90-0
- Pyrolin
Catalog No.:BCN6902
CAS No.:95-71-6
- 2-(Morpholinodithio)benzothiazole
Catalog No.:BCC8484
CAS No.:95-32-9
- 2-Benzothiazolyl diethyldithiocarbamate
Catalog No.:BCC8558
CAS No.:95-30-7
- Chlorzoxazone
Catalog No.:BCC4650
CAS No.:95-25-0
- 2-Amino-6-chlorobenzothiazole
Catalog No.:BCC8539
CAS No.:95-24-9
- 5-Amino-1,3-dihydro-2H-benzimidazol-2-one
Catalog No.:BCC8728
CAS No.:95-23-8
- trans-4-Hydroxy-2-nonenoic acid
Catalog No.:BCN7959
CAS No.:95087-42-6
- PF-670462
Catalog No.:BCC1856
CAS No.:950912-80-8
- 2'-Deoxyuridine
Catalog No.:BCC8278
CAS No.:951-78-0
- Boc-N-Me-Tyr-OH.DCHA
Catalog No.:BCC3355
CAS No.:95105-25-2
- Desformylflustrabromine hydrochloride
Catalog No.:BCC7651
CAS No.:951322-11-5
- Artemetin acetate
Catalog No.:BCN4501
CAS No.:95135-98-1
- DY131
Catalog No.:BCC1539
CAS No.:95167-41-2
- Herpetone
Catalog No.:BCN2812
CAS No.:951677-22-8
- Fmoc-Lys(Me,Boc)-OH
Catalog No.:BCC2566
CAS No.:951695-85-5
- Sibiricin
Catalog No.:BCN4502
CAS No.:95188-34-4
- PF-477736
Catalog No.:BCC4421
CAS No.:952021-60-2
- Parishin E
Catalog No.:BCN3814
CAS No.:952068-57-4
Crystal structure of the FLT3 kinase domain bound to the inhibitor Quizartinib (AC220).[Pubmed:25837374]
PLoS One. 2015 Apr 2;10(4):e0121177.
More than 30% of acute myeloid leukemia (AML) patients possess activating mutations in the receptor tyrosine kinase FMS-like tyrosine kinase 3 or FLT3. A small-molecule inhibitor of FLT3 (known as quizartinib or AC220) that is currently in clinical trials appears promising for the treatment of AML. Here, we report the co-crystal structure of the kinase domain of FLT3 in complex with quizartinib. FLT3 with quizartinib bound adopts an "Abl-like" inactive conformation with the activation loop stabilized in the "DFG-out" orientation and folded back onto the kinase domain. This conformation is similar to that observed for the uncomplexed intracellular domain of FLT3 as well as for related receptor tyrosine kinases, except for a localized induced fit in the activation loop. The co-crystal structure reveals the interactions between quizartinib and the active site of FLT3 that are key for achieving its high potency against both wild-type FLT3 as well as a FLT3 variant observed in many AML patients. This co-complex further provides a structural rationale for quizartinib-resistance mutations.
Quizartinib (AC220) is a potent second generation class III tyrosine kinase inhibitor that displays a distinct inhibition profile against mutant-FLT3, -PDGFRA and -KIT isoforms.[Pubmed:23497317]
Mol Cancer. 2013 Mar 7;12:19.
BACKGROUND: Activating mutations of class III receptor tyrosine kinases (RTK) FLT3, PDGFR and KIT are associated with multiple human neoplasms including hematologic malignancies, for example: systemic mast cell disorders (KIT), non-CML myeloproliferative neoplasms (PDGFR) and subsets of acute leukemias (FLT3 and KIT). First generation tyrosine kinase inhibitors (TKI) are rapidly being integrated into routine cancer care. However, the expanding spectrum of TK-mutations, bioavailability issues and the emerging problem of primary or secondary TKI-therapy resistance have lead to the search for novel second generation TKIs to improve target potency and to overcome resistant clones.Quizartinib was recently demonstrated to be a selective FLT3 inhibitor with excellent pharmacokinetics and promising in vivo activity in a phase II study for FLT3 ITD + AML patients. In vitro kinase assays have suggested that in addition to FLT3, quizartinib also targets related class III RTK isoforms. METHODS: Various FLT3 or KIT leukemia cell lines and native blasts were used to determine the antiproliferative and proapoptotic efficacy of quizartinib. To better compare differences between the mutant kinase isoforms, we generated an isogenic BaF3 cell line expressing different FLT3, KIT or BCR/ABL isoforms. Using immunoblotting, we examined the effects of quizartinib on activation of mutant KIT or FLT3 isoforms. RESULTS: Kinase inhibition of (mutant) KIT, PDGFR and FLT3 isoforms by quizartinib leads to potent inhibition of cellular proliferation and induction of apoptosis in in vitro leukemia models as well as in native leukemia blasts treated ex vivo. However, the sensitivity patterns vary widely depending on the underlying (mutant)-kinase isoform, with some isoforms being relatively insensitive to this agent (e.g. FLT3 D835V and KIT codon D816 mutations). Evaluation of sensitivities in an isogenic cellular background confirms a direct association with the underlying mutant-TK isoform--which is further validated by immunoblotting experiments demonstrating kinase inhibition consistent with the cellular sensitivity/resistance to quizartinib. CONCLUSION: Quizartinib is a potent second-generation class III receptor TK-inhibitor--but specific, mutation restricted spectrum of activity may require mutation screening prior to therapy.


