HerpetoneCAS# 951677-22-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

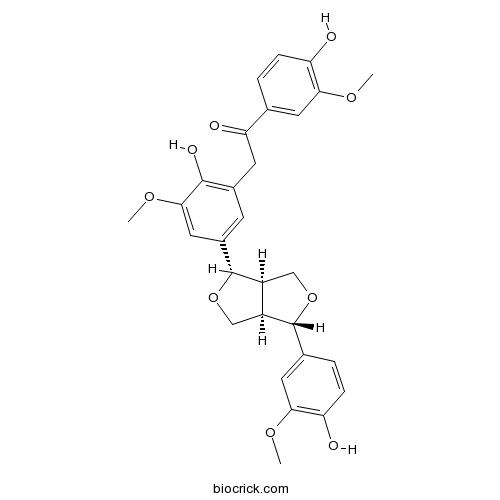
| Cas No. | 951677-22-8 | SDF | Download SDF |
| PubChem ID | 102004856 | Appearance | Powder |
| Formula | C29H30O9 | M.Wt | 522.54 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-[5-[(3S,3aR,6S,6aR)-3-(4-hydroxy-3-methoxyphenyl)-1,3,3a,4,6,6a-hexahydrofuro[3,4-c]furan-6-yl]-2-hydroxy-3-methoxyphenyl]-1-(4-hydroxy-3-methoxyphenyl)ethanone | ||
| SMILES | COC1=CC(=CC(=C1O)CC(=O)C2=CC(=C(C=C2)O)OC)C3C4COC(C4CO3)C5=CC(=C(C=C5)O)OC | ||
| Standard InChIKey | NKRVXSJMQLQTTM-UGOBFYTOSA-N | ||
| Standard InChI | InChI=1S/C29H30O9/c1-34-24-10-15(4-6-21(24)30)23(32)9-17-8-18(12-26(36-3)27(17)33)29-20-14-37-28(19(20)13-38-29)16-5-7-22(31)25(11-16)35-2/h4-8,10-12,19-20,28-31,33H,9,13-14H2,1-3H3/t19-,20-,28+,29+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Herpetone shows anti-hepatitis B virus (HBV) activity. 2. Herpetone exhibits protective effects on CCl(4)-induced hepatocyte injury. |
| Targets | HBV |

Herpetone Dilution Calculator

Herpetone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9137 mL | 9.5686 mL | 19.1373 mL | 38.2746 mL | 47.8432 mL |
| 5 mM | 0.3827 mL | 1.9137 mL | 3.8275 mL | 7.6549 mL | 9.5686 mL |
| 10 mM | 0.1914 mL | 0.9569 mL | 1.9137 mL | 3.8275 mL | 4.7843 mL |
| 50 mM | 0.0383 mL | 0.1914 mL | 0.3827 mL | 0.7655 mL | 0.9569 mL |
| 100 mM | 0.0191 mL | 0.0957 mL | 0.1914 mL | 0.3827 mL | 0.4784 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- DY131
Catalog No.:BCC1539
CAS No.:95167-41-2
- Artemetin acetate
Catalog No.:BCN4501
CAS No.:95135-98-1
- Desformylflustrabromine hydrochloride
Catalog No.:BCC7651
CAS No.:951322-11-5
- Boc-N-Me-Tyr-OH.DCHA
Catalog No.:BCC3355
CAS No.:95105-25-2
- 2'-Deoxyuridine
Catalog No.:BCC8278
CAS No.:951-78-0
- PF-670462
Catalog No.:BCC1856
CAS No.:950912-80-8
- trans-4-Hydroxy-2-nonenoic acid
Catalog No.:BCN7959
CAS No.:95087-42-6
- Quizartinib (AC220)
Catalog No.:BCC2548
CAS No.:950769-58-1
- PCI-34051
Catalog No.:BCC2148
CAS No.:950762-95-5
- B-Raf IN 1
Catalog No.:BCC5439
CAS No.:950736-05-7
- 6-O-Methacryloyltrilobolide
Catalog No.:BCN7599
CAS No.:950685-51-5
- Gemcitabine
Catalog No.:BCC3784
CAS No.:95058-81-4
- Fmoc-Lys(Me,Boc)-OH
Catalog No.:BCC2566
CAS No.:951695-85-5
- Sibiricin
Catalog No.:BCN4502
CAS No.:95188-34-4
- PF-477736
Catalog No.:BCC4421
CAS No.:952021-60-2
- Parishin E
Catalog No.:BCN3814
CAS No.:952068-57-4
- Atovaquone
Catalog No.:BCC4890
CAS No.:95233-18-4
- Neocaesalpin L
Catalog No.:BCN7650
CAS No.:952473-86-8
- 11-Oxomogroside III
Catalog No.:BCN3169
CAS No.:952481-53-7
- Hydrangenoside A dimethyl acetal
Catalog No.:BCN4553
CAS No.:952485-00-6
- Yadanzioside F
Catalog No.:BCN6406
CAS No.:95258-11-0
- Yadanziolide C
Catalog No.:BCN6719
CAS No.:95258-12-1
- Yadanziolide B
Catalog No.:BCN6720
CAS No.:95258-13-2
- Yadanziolide A
Catalog No.:BCN6721
CAS No.:95258-14-3
Anti-HBV Activities of Three Compounds Extracted and Purified from Herpetospermum Seeds.[Pubmed:28035986]
Molecules. 2016 Dec 27;22(1). pii: molecules22010014.
The goal of this research was to evaluate the anti-hepatitis B virus (HBV) activities of three compounds extracted and purified from Herpetospermum seeds (HS) on HepG2.2.15 cells. Herpetin (HPT), Herpetone (HPO), and herpetfluorenone (HPF) were isolated from HS and identified using HR-ESI-MS and NMR. Different concentrations of the drugs were added to the HepG2.2.15 cells. Cell toxicity was observed with an MTT assay, cell culture supernatants were collected, and HBsAg and HBeAg were detected by ELISA. The content of HBV DNA was determined via quantitative polymerase chain reaction (PCR) with fluorescent probes. The 50% toxicity concentration (TC50) of HPF was 531.48 mug/mL, suggesting that this species is less toxic than HPT and HPO. HPT and HPF showed more potent antiviral activities than HPO. The 50% inhibition concentration (IC50) values of HPF on HBsAg and HBeAg were 176.99 and 134.53 mug/mL, respectively, and the corresponding therapeutic index (TI) values were 2.66 and 3.49, respectively. HPT and HPF were shown to significantly reduce the level of HBV DNA in the HepG2.2.15 culture medium compared to the negative control. This initial investigation of the anti-HBV constituents of HS yielded three compounds that revealed a synergistic effect of multiple components in the ethnopharmacological use of HS.
Two new coumarins from Herpetospermum caudigerum.[Pubmed:18239307]
Chem Pharm Bull (Tokyo). 2008 Feb;56(2):192-3.
The ethyl acetate extract from the seeds of Herpetospermum caudigerum was found to show protective effects on carbon tetrachloride (CCl(4)) and thioacetamide (TAA)-induced acute hepatic injuries in mice. From the ethyl acetate extract, two new coumarins, herpetolide A (1) and herpetolide B (2), along with four known compounds, Herpetone (3), dehydrodiconiferyl alcohol (4), 2,4-dihydroxypyrimidine (5) and stigmasterol (6) were isolated. The structures of the new coumarins were elucidated on the basis of chemical and physicochemical evidences. Herpetone exhibited protective effects on CCl(4)-induced hepatocyte injury.


