B-Raf IN 1CAS# 950736-05-7 |
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
- Myelin Basic Protein (68-82), guinea pig
Catalog No.:BCC1020
CAS No.:98474-59-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 950736-05-7 | SDF | Download SDF |
| PubChem ID | 24884503 | Appearance | Powder |
| Formula | C29H24F3N5O | M.Wt | 515.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 53 mg/mL (102.81 mM) *"≥" means soluble, but saturation unknown. | ||
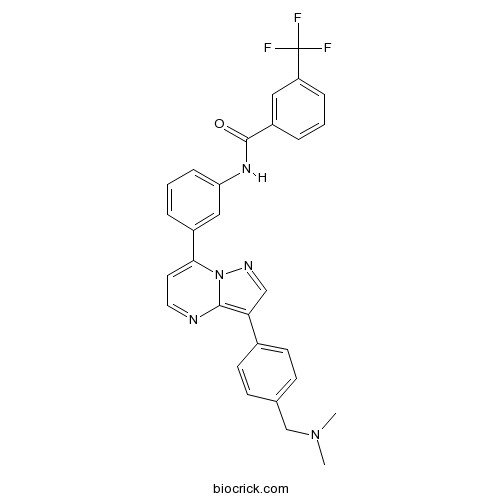
| Chemical Name | N-[3-[3-[4-[(dimethylamino)methyl]phenyl]pyrazolo[1,5-a]pyrimidin-7-yl]phenyl]-3-(trifluoromethyl)benzamide | ||
| SMILES | CN(C)CC1=CC=C(C=C1)C2=C3N=CC=C(N3N=C2)C4=CC(=CC=C4)NC(=O)C5=CC(=CC=C5)C(F)(F)F | ||
| Standard InChIKey | AIWJVLQNYNCDSL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C29H24F3N5O/c1-36(2)18-19-9-11-20(12-10-19)25-17-34-37-26(13-14-33-27(25)37)21-5-4-8-24(16-21)35-28(38)22-6-3-7-23(15-22)29(30,31)32/h3-17H,18H2,1-2H3,(H,35,38) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

B-Raf IN 1 Dilution Calculator

B-Raf IN 1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9398 mL | 9.6988 mL | 19.3975 mL | 38.795 mL | 48.4938 mL |
| 5 mM | 0.388 mL | 1.9398 mL | 3.8795 mL | 7.759 mL | 9.6988 mL |
| 10 mM | 0.194 mL | 0.9699 mL | 1.9398 mL | 3.8795 mL | 4.8494 mL |
| 50 mM | 0.0388 mL | 0.194 mL | 0.388 mL | 0.7759 mL | 0.9699 mL |
| 100 mM | 0.0194 mL | 0.097 mL | 0.194 mL | 0.388 mL | 0.4849 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
B-Raf IN 1 is a highlt potent and selective B-Raf inhibitor with IC50 of 24 nM; equipotent against c-Raf (IC50= 25 nM). IC50 value: 24 nM (B-Raf); 25 nM (C-Raf) [1] Target: B-Raf/C-Raf inhibitor Compound 10n (B-Raf IN 1) was equipotent against c-Raf (IC50: 0.025 lM) Moderate selectivity was observed for compound 10n versus p38a (IC50: 0.216 lM) and CAMKII (IC50: 0.822 lM), while high selectivity was observed versus CDK2, CDK4, PKCa, IKKb, JNK1, MK2, PKA, Src, MKK6, PLK1, p70S6K, PI3 Ka, and PDK1 (IC50s: >2 lM).
References:
[1]. Berger DM, et al. Non-hinge-binding pyrazolo[1,5-a]pyrimidines as potent B-Raf kinase inhibitors. Bioorg Med Chem Lett. 2009 Dec 1;19(23):6519-23.
- 6-O-Methacryloyltrilobolide
Catalog No.:BCN7599
CAS No.:950685-51-5
- Gemcitabine
Catalog No.:BCC3784
CAS No.:95058-81-4
- Liproxstatin-1
Catalog No.:BCC5651
CAS No.:950455-15-9
- Erianin
Catalog No.:BCN2350
CAS No.:95041-90-0
- Pyrolin
Catalog No.:BCN6902
CAS No.:95-71-6
- 2-(Morpholinodithio)benzothiazole
Catalog No.:BCC8484
CAS No.:95-32-9
- 2-Benzothiazolyl diethyldithiocarbamate
Catalog No.:BCC8558
CAS No.:95-30-7
- Chlorzoxazone
Catalog No.:BCC4650
CAS No.:95-25-0
- 2-Amino-6-chlorobenzothiazole
Catalog No.:BCC8539
CAS No.:95-24-9
- 5-Amino-1,3-dihydro-2H-benzimidazol-2-one
Catalog No.:BCC8728
CAS No.:95-23-8
- Thianaphthene
Catalog No.:BCC9178
CAS No.:95-15-8
- Quercetin-3-o-rutinose
Catalog No.:BCN3404
CAS No.:949926-49-2
- PCI-34051
Catalog No.:BCC2148
CAS No.:950762-95-5
- Quizartinib (AC220)
Catalog No.:BCC2548
CAS No.:950769-58-1
- trans-4-Hydroxy-2-nonenoic acid
Catalog No.:BCN7959
CAS No.:95087-42-6
- PF-670462
Catalog No.:BCC1856
CAS No.:950912-80-8
- 2'-Deoxyuridine
Catalog No.:BCC8278
CAS No.:951-78-0
- Boc-N-Me-Tyr-OH.DCHA
Catalog No.:BCC3355
CAS No.:95105-25-2
- Desformylflustrabromine hydrochloride
Catalog No.:BCC7651
CAS No.:951322-11-5
- Artemetin acetate
Catalog No.:BCN4501
CAS No.:95135-98-1
- DY131
Catalog No.:BCC1539
CAS No.:95167-41-2
- Herpetone
Catalog No.:BCN2812
CAS No.:951677-22-8
- Fmoc-Lys(Me,Boc)-OH
Catalog No.:BCC2566
CAS No.:951695-85-5
- Sibiricin
Catalog No.:BCN4502
CAS No.:95188-34-4
Modification, biological evaluation and 3D QSAR studies of novel 2-(1,3-diaryl- 4,5-dihydro-1H-pyrazol-5-yl)phenol derivatives as inhibitors of B-Raf kinase.[Pubmed:24827980]
PLoS One. 2014 May 14;9(5):e95702.
A series of novel 2-(1,3-diaryl- 4,5-dihydro-1H-pyrazol-5-yl)phenol derivatives (C1-C24) have been synthesized. The B-Raf inhibitory activity and anti-proliferation activity of these compounds have been tested. Compound C6 displayed the most potent biological activity against B-RafV600E (IC50 = 0.15 microM) and WM266.4 human melanoma cell line (GI50 = 1.75 microM), being comparable with the positive control (Vemurafenib and Erlotinib) and more potent than our previous best compounds. The docking simulation was performed to analyze the probable binding models and poses while the QSAR model was built to check the previous work as well as to introduce new directions. This work aimed at seeking more potent inhibitors as well as discussing some previous findings. As a result, the introduction of ortho-hydroxyl group on 4,5-dihydro-1H-pyrazole skeleton did reinforce the anti-tumor activity while enlarging the group on N-1 of pyrazoline was also helpful.
Mutant B-RAF-Mcl-1 survival signaling depends on the STAT3 transcription factor.[Pubmed:23455323]
Oncogene. 2014 Feb 27;33(9):1158-66.
Approximately 50% of melanomas depend on mutant B-RAF for proliferation, metastasis and survival. The inhibition of oncogenic B-RAF with highly targeted compounds has produced remarkable albeit short-lived clinical responses in B-RAF mutant melanoma patients. Reactivation of signaling downstream of B-RAF is frequently associated with acquired resistance to B-RAF inhibitors, and the identification of B-RAF targets may provide new strategies for managing melanoma. Oncogenic B-RAF(V600E) is known to promote the stabilizing phosphorylation of the anti-apoptotic protein Mcl-1, implicated in melanoma survival and chemoresistance. We now show that B-RAF(V600E) signaling also induces the transcription of Mcl-1 in melanocytes and melanoma. We demonstrate that activation of STAT3 serine-727 and tyrosine-705 phosphorylations is promoted by B-RAF(V600E) activity and that the Mcl-1 promoter is dependent on a STAT consensus-site for B-RAF-mediated activation. Consequently, suppression of STAT3 activity disrupted B-RAF(V600E)-mediated induction of Mcl-1 and reduced melanoma cell survival. We propose that STAT3 has a central role in the survival and contributes to chemoresistance of B-RAF(V600E) melanoma.
B-RAF and its novel negative regulator reticulocalbin 1 (RCN1) modulates cardiomyocyte hypertrophy.[Pubmed:24492844]
Cardiovasc Res. 2014 Apr 1;102(1):88-96.
AIM: Activation of the kinase RAF and its downstream targets leads to cardiomyocyte hypertrophy. It has been hypothesized that B-RAF might be the main activator of MEK in various cell types. Therefore, the aim of this study was to investigate the role of B-RAF and its modulating factors in cardiomyocyte hypertrophy. METHODS AND RESULTS: Neonatal rat cardiomyocytes were pre-treated with and without the specific B-RAF inhibitor SB590885 and then stimulated with phenylephrine to induce hypertrophy. Inhibition of B-RAF completely impeded the hypertrophic response and led to a significant reduction of MEK1/2 phosphorylation. By applying a eukaryotic cDNA expression screen, based on a dual-luciferase reporter assay for B-RAF activity measurement, we identified RCN1 as a new negative modulator of B-RAF activity. Adenovirus-mediated overexpression of reticulocalbin 1 (RCN1) completely impeded phenylephrine-induced hypertrophy and led to significantly reduced MEK1/2 phosphorylation. Conversely, adenoviral knockdown of RCN1 with a specific synthetic miRNA induced cardiomyocyte hypertrophy and significantly increased MEK1/2 phosphorylation. CONCLUSIONS: In summary, our results show that the inhibition of B-RAF abolishes cardiomyocyte hypertrophy and we identified RCN1 as novel negative modulator of cardiomyocyte hypertrophy by inhibition of the mitogen-activated protein kinase signalling cascade. Our results show that B-RAF kinase activity is essential for cardiac hypertrophy and RCN1, its newly identified negative regulator, abolishes hypertrophic response of cardiomyocytes in vitro.
Design, modification and 3D QSAR studies of novel 2,3-dihydrobenzo[b][1,4]dioxin-containing 4,5-dihydro-1H-pyrazole derivatives as inhibitors of B-Raf kinase.[Pubmed:22985962]
Bioorg Med Chem. 2012 Oct 15;20(20):6048-58.
Two series of novel 2,3-dihydrobenzo[b][1,4]dioxin-containing 4,5-dihydro-1H-pyrazole derivatives C1-C15 and D1-D15 have been synthesized and evaluated for their B-Raf inhibitory and anti-proliferation activities. Compound C14 ((3-(4-bromophenyl)-5-(2-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)(2,3-dihydrobe nzo[b][1,4]dioxin-6-yl)methanone) showed the most potent biological activity against B-Raf(V600E) (IC(50) = 0.11 muM) and WM266.4 human melanoma cell line (GI(50) = 0.58 muM), being comparable with the positive control Erlotinib and more potent than our previous best compound, while D10 ((2,3-dihydrobenzo[b][1,4]dioxin-2-yl)(5-(3-fluorophenyl)-3-phenyl-4,5-dihydro-1H -pyrazol-1-yl)methanone) performed the best in the D series (IC(50) = 1.70 muM; GI(50) = 1.45 muM). The docking simulation was performed to analyze the probable binding models and poses and the QSAR model was built for reasonable design of B-Raf inhibitors in future. The introduction of 2,3-dihydrobenzo[b][1,4]dioxin structure reinforced the combination of our compounds and the receptor, resulting in progress of bioactivity.


