DY131ERRγ agonist CAS# 95167-41-2 |
- Finasteride acetate
Catalog No.:BCC4204
CAS No.:222989-99-3
- Finasteride
Catalog No.:BCC2491
CAS No.:98319-26-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 95167-41-2 | SDF | Download SDF |
| PubChem ID | 5410903 | Appearance | Powder |
| Formula | C18H21N3O2 | M.Wt | 311.38 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (160.58 mM; Need ultrasonic) | ||
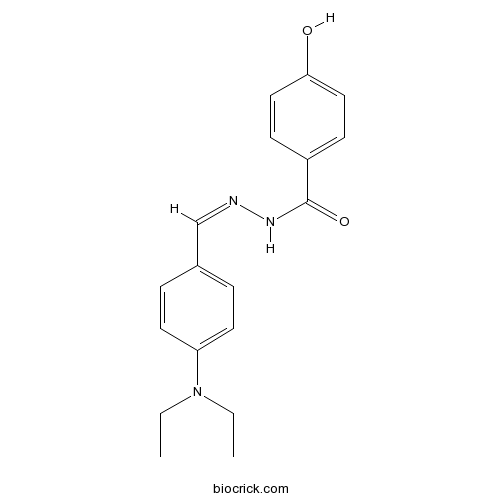
| Chemical Name | N-[(Z)-[4-(diethylamino)phenyl]methylideneamino]-4-hydroxybenzamide | ||
| SMILES | CCN(CC)C1=CC=C(C=C1)C=NNC(=O)C2=CC=C(C=C2)O | ||
| Standard InChIKey | WLKOCYWYAWBGKY-UYRXBGFRSA-N | ||
| Standard InChI | InChI=1S/C18H21N3O2/c1-3-21(4-2)16-9-5-14(6-10-16)13-19-20-18(23)15-7-11-17(22)12-8-15/h5-13,22H,3-4H2,1-2H3,(H,20,23)/b19-13- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Novel selective agonist at estrogen-related receptors ERRβ and ERRγ. Displays minimal activity at ERRα, ERα and ERβ at concentrations up to 30 μM. |

DY131 Dilution Calculator

DY131 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2115 mL | 16.0576 mL | 32.1151 mL | 64.2302 mL | 80.2878 mL |
| 5 mM | 0.6423 mL | 3.2115 mL | 6.423 mL | 12.846 mL | 16.0576 mL |
| 10 mM | 0.3212 mL | 1.6058 mL | 3.2115 mL | 6.423 mL | 8.0288 mL |
| 50 mM | 0.0642 mL | 0.3212 mL | 0.6423 mL | 1.2846 mL | 1.6058 mL |
| 100 mM | 0.0321 mL | 0.1606 mL | 0.3212 mL | 0.6423 mL | 0.8029 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: N/A
DY131 is an agonist ligand for estrogen-related receptors ERRβ/γ. Estrogen receptor-related receptors (ERR) are orphan nuclear receptors constitutively activated without estrogen binding. ERRs may be involved in similar ER-mediated regulatory pathways and modulate estrogen responsiveness in certain target cells. The ERR subtypes, ERRβ/γ, are coexpressed in normal human prostatic epithelial cells and exhibit reduced expression in many prostate cancer cell lines.
In vitro: DY131 was evaluated for its selectivity and efficacy in modulating the transcriptional activity of ERRα/β/γ and ERβ/γ. CV-1 cells were transfected with reporter constructs or expression vectors and the fold activation of the reporter construct was determined at different concentrations of DY131. DY131 was found to be not able to activate the reporter construct or ERRα at any of the tested concentrations. In contrast, DY131 led to three- to fourfold activation of ERRβ at concentrations of 10-30 μM. Activity on ERRγ was more pronounced with five-fold activation at 3 μM and maximal 6.6-fold activity observed at 30 μM. Thus, DY131 was an selective ERRβ/γ ligand displaying preferential selectivity for ERRγ at lower concentrations [1].
In vivo: Currently, there is no animal in vivo data available for DY131 and its analogs.
Clinical trial: So far, no clinical study has been reported for DY131 and its analogs.
Reference:
[1] Yu DD,Forman BM. Identification of an agonist ligand for estrogen-related receptors ERRbeta/gamma. Bioorg Med Chem Lett.2005 Mar 1;15(5):1311-3.
- Artemetin acetate
Catalog No.:BCN4501
CAS No.:95135-98-1
- Desformylflustrabromine hydrochloride
Catalog No.:BCC7651
CAS No.:951322-11-5
- Boc-N-Me-Tyr-OH.DCHA
Catalog No.:BCC3355
CAS No.:95105-25-2
- 2'-Deoxyuridine
Catalog No.:BCC8278
CAS No.:951-78-0
- PF-670462
Catalog No.:BCC1856
CAS No.:950912-80-8
- trans-4-Hydroxy-2-nonenoic acid
Catalog No.:BCN7959
CAS No.:95087-42-6
- Quizartinib (AC220)
Catalog No.:BCC2548
CAS No.:950769-58-1
- PCI-34051
Catalog No.:BCC2148
CAS No.:950762-95-5
- B-Raf IN 1
Catalog No.:BCC5439
CAS No.:950736-05-7
- 6-O-Methacryloyltrilobolide
Catalog No.:BCN7599
CAS No.:950685-51-5
- Gemcitabine
Catalog No.:BCC3784
CAS No.:95058-81-4
- Liproxstatin-1
Catalog No.:BCC5651
CAS No.:950455-15-9
- Herpetone
Catalog No.:BCN2812
CAS No.:951677-22-8
- Fmoc-Lys(Me,Boc)-OH
Catalog No.:BCC2566
CAS No.:951695-85-5
- Sibiricin
Catalog No.:BCN4502
CAS No.:95188-34-4
- PF-477736
Catalog No.:BCC4421
CAS No.:952021-60-2
- Parishin E
Catalog No.:BCN3814
CAS No.:952068-57-4
- Atovaquone
Catalog No.:BCC4890
CAS No.:95233-18-4
- Neocaesalpin L
Catalog No.:BCN7650
CAS No.:952473-86-8
- 11-Oxomogroside III
Catalog No.:BCN3169
CAS No.:952481-53-7
- Hydrangenoside A dimethyl acetal
Catalog No.:BCN4553
CAS No.:952485-00-6
- Yadanzioside F
Catalog No.:BCN6406
CAS No.:95258-11-0
- Yadanziolide C
Catalog No.:BCN6719
CAS No.:95258-12-1
- Yadanziolide B
Catalog No.:BCN6720
CAS No.:95258-13-2
Antimitotic activity of DY131 and the estrogen-related receptor beta 2 (ERRbeta2) splice variant in breast cancer.[Pubmed:27363015]
Oncotarget. 2016 Jul 26;7(30):47201-47220.
Breast cancer remains a leading cause of cancer-related death in women, and triple negative breast cancer (TNBC) lacks clinically actionable therapeutic targets. Death in mitosis is a tumor suppressive mechanism that occurs in cancer cells experiencing a defective M phase. The orphan estrogen-related receptor beta (ERRbeta) is a key reprogramming factor in murine embryonic and induced pluripotent stem cells. In primates, ERRbeta is alternatively spliced to produce several receptor isoforms. In cellular models of glioblastoma, short form (ERRbetasf) and beta2 (ERRbeta2) splice variants differentially regulate cell cycle progression in response to the synthetic agonist DY131, with ERRbeta2 driving arrest in G2/M.The goals of the present study are to determine the cellular function(s) of ligand-activated ERRbeta splice variants in breast cancer and evaluate the potential of DY131 to serve as an antimitotic agent, particularly in TNBC. DY131 inhibits growth in a diverse panel of breast cancer cell lines, causing cell death that involves the p38 stress kinase pathway and a bimodal cell cycle arrest. ERRbeta2 facilitates the block in G2/M, and DY131 delays progression from prophase to anaphase. Finally, ERRbeta2 localizes to centrosomes and DY131 causes mitotic spindle defects. Targeting ERRbeta2 may therefore be a promising therapeutic strategy in breast cancer.
Identification of an agonist ligand for estrogen-related receptors ERRbeta/gamma.[Pubmed:15713377]
Bioorg Med Chem Lett. 2005 Mar 1;15(5):1311-3.
In order to develop agonist ligands that are specific for the estrogen-related receptors ERRbeta/gamma, a hydrazone with a 4-hydroxy group at one phenyl ring and a 4-diethylamino moiety at the other phenyl ring was synthesized. We demonstrate that compound 3 (DY131; N'-{(1E)-[4-(diethylamino)phenyl]methylene}-4-hydroxybenzohydrazide) effectively and selectively activates ERRbeta/gamma. DY131 had no effect on the structurally related receptors ERRalpha or the estrogen receptors alpha and beta (ERalpha/beta). This work defines a convenient synthesis for a novel and selective pharmacologic tool that can be used to elucidate the biological activities of ERRbeta/gamma.


