Tandutinib (MLN518)FLT3 inhibitor,potent and selective CAS# 387867-13-2 |
- Levomefolic acid
Catalog No.:BCC1703
CAS No.:31690-09-2
- Megestrol Acetate
Catalog No.:BCC4365
CAS No.:595-33-5
- Dienogest
Catalog No.:BCC4489
CAS No.:65928-58-7
- Drospirenone
Catalog No.:BCC4493
CAS No.:67392-87-4
- Nestoron
Catalog No.:BCC1797
CAS No.:7759-35-5
- Levonorgestrel
Catalog No.:BCC4792
CAS No.:797-63-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 387867-13-2 | SDF | Download SDF |
| PubChem ID | 3038522 | Appearance | Powder |
| Formula | C31H42N6O4 | M.Wt | 562.72 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | MLN518; CT53518 | ||
| Solubility | DMSO : 50 mg/mL (88.86 mM; Need ultrasonic) | ||
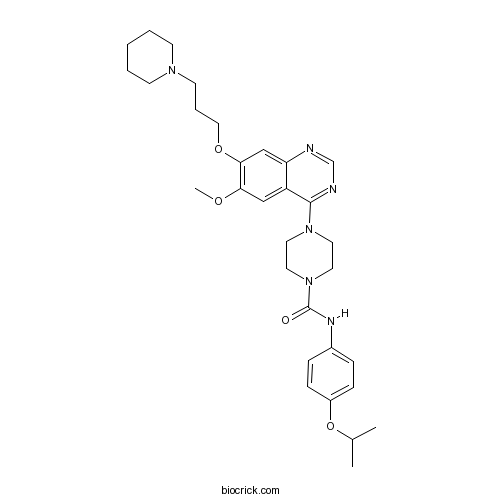
| Chemical Name | 4-[6-methoxy-7-(3-piperidin-1-ylpropoxy)quinazolin-4-yl]-N-(4-propan-2-yloxyphenyl)piperazine-1-carboxamide | ||
| SMILES | CC(C)OC1=CC=C(C=C1)NC(=O)N2CCN(CC2)C3=NC=NC4=CC(=C(C=C43)OC)OCCCN5CCCCC5 | ||
| Standard InChIKey | UXXQOJXBIDBUAC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C31H42N6O4/c1-23(2)41-25-10-8-24(9-11-25)34-31(38)37-17-15-36(16-18-37)30-26-20-28(39-3)29(21-27(26)32-22-33-30)40-19-7-14-35-12-5-4-6-13-35/h8-11,20-23H,4-7,12-19H2,1-3H3,(H,34,38) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tandutinib (MLN518, CT53518) is a potent antagonist of FLT3 with an IC50 value of 0.22 μM. | ||||||
| Targets | c-Kit | PDGFRβ | FLT3 | CSF-1R | KDR | ||
| IC50 | 0.17 μM | 0.20 μM | 0.22 μM | 3.43 μM | >30 μM | ||

Tandutinib (MLN518) Dilution Calculator

Tandutinib (MLN518) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7771 mL | 8.8854 mL | 17.7708 mL | 35.5417 mL | 44.4271 mL |
| 5 mM | 0.3554 mL | 1.7771 mL | 3.5542 mL | 7.1083 mL | 8.8854 mL |
| 10 mM | 0.1777 mL | 0.8885 mL | 1.7771 mL | 3.5542 mL | 4.4427 mL |
| 50 mM | 0.0355 mL | 0.1777 mL | 0.3554 mL | 0.7108 mL | 0.8885 mL |
| 100 mM | 0.0178 mL | 0.0889 mL | 0.1777 mL | 0.3554 mL | 0.4443 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tandutinib (MLN518, CT53518) is a novel, selective and small-molecule inhibitor of FLT3 with IC50 value of 0.22μM [1].
Tandutinib (MLN518) has been reported to inhibit FLT3, PDGFR and c-Kit in in vitro kinase assays with IC50 values of 0.22μM, 0.20μM and 0.17μM, respectively. In addition, Tandutinib (MLN518) has been revealed to inhibit wild-type FLT3 or W51 tyrosine phosphorylation with an IC50 value of 30–100 nM. Furthermore, Tandutinib (MLN518) has shown the cell antiproliferation of the FLT3-ITD-positive cells (Molm-13 and Molm-14 cells) with an IC50 value of 10 nM, whereas the FLT3-ITD-negative cells ( THP-1, KG-1, and RS4 cells) were resistant, requiring 1000-fold higher concentrations to inhibit cell growth [1]
References:
[1] Kelly LM1, Yu JC, Boulton CL, Apatira M, Li J, Sullivan CM, Williams I, Amaral SM, Curley DP, Duclos N, Neuberg D, Scarborough RM, Pandey A, Hollenbach S, Abe K, Lokker NA, Gilliland DG, Giese NA. CT53518, a novel selective FLT3 antagonist for the treatment of acute myelogenous leukemia (AML). Cancer Cell. 2002 Jun;1(5):421-32.
- Tectoruside
Catalog No.:BCN8262
CAS No.:38784-73-5
- Muristerone A
Catalog No.:BCC2397
CAS No.:38778-30-2
- Tetrahydroisocucurbitacin I
Catalog No.:BCN7874
CAS No.:3877-89-2
- Cucurbitacin D
Catalog No.:BCN2355
CAS No.:3877-86-9
- Swazine
Catalog No.:BCN2143
CAS No.:38763-74-5
- Worenine
Catalog No.:BCN2557
CAS No.:38763-29-0
- Triptolide
Catalog No.:BCN5984
CAS No.:38748-32-2
- 3-Epibetulinic acid
Catalog No.:BCN8531
CAS No.:38736-77-5
- Seneciphylline N-oxide
Catalog No.:BCN5439
CAS No.:38710-26-8
- Preskimmianine
Catalog No.:BCN6667
CAS No.:38695-41-9
- Groenlandicine
Catalog No.:BCN8189
CAS No.:38691-95-1
- DAPK Substrate Peptide
Catalog No.:BCC2400
CAS No.:386769-53-5
- Lapatinib Ditosylate
Catalog No.:BCC2083
CAS No.:388082-78-8
- Emodin-1-O-glucoside
Catalog No.:BCN8512
CAS No.:38840-23-2
- Tetrahydroepiberberine
Catalog No.:BCN2649
CAS No.:38853-67-7
- PSN 375963 hydrochloride
Catalog No.:BCC7663
CAS No.:388575-52-8
- CK 869
Catalog No.:BCC6347
CAS No.:388592-44-7
- Z-Glu(OtBu)-OH
Catalog No.:BCC2776
CAS No.:3886-08-6
- Halaminol A
Catalog No.:BCN1788
CAS No.:389125-56-8
- Halaminol B
Catalog No.:BCN1748
CAS No.:389125-59-1
- Halaminol C
Catalog No.:BCN1787
CAS No.:389125-68-2
- erythro-Guaiacylglycerol
Catalog No.:BCN5440
CAS No.:38916-91-5
- Isodeoxyelephantopin
Catalog No.:BCN4638
CAS No.:38927-54-7
- SBHA
Catalog No.:BCC2425
CAS No.:38937-66-5
Tandutinib (MLN518) reverses multidrug resistance by inhibiting the efflux activity of the multidrug resistance protein 7 (ABCC10).[Pubmed:23525656]
Oncol Rep. 2013 Jun;29(6):2479-85.
It is well established that ATP-binding cassette (ABC) transporter-mediated multidrug resistance (MDR) is one of the major mechanisms that causes resistance to antineoplastic drugs in cancer cells. ABC transporters can significantly decrease the intracellular concentration of antineoplastic drugs by increasing their efflux, thereby lowering their cytotoxic activity. One of these transporters, the multidrug resistance protein 7 (MRP7/ABCC10), has already been shown to produce resistance to antineoplastic drugs by increasing the efflux of the drugs. In the present study, we investigated whether tandutinib, an FMS-like tyrosine kinase 3 (FLT3) inhibitor, has the potential to reverse MRP7-mediated MDR. Our results revealed that tandutinib significantly enhanced the sensitivity of MRP7-transfected HEK293 cells to the 2 established MRP7 substrates, paclitaxel and vincristine, whereas there was less or no effect on the control vector-transfected HEK293 cells. [(3)H]-paclitaxel accumulation and efflux studies demonstrated that tandutinib increased the intracellular accumulation of [(3)H]-paclitaxel and inhibited the efflux of [(3)H]-paclitaxel from HEK-MRP7 cells. In addition, western blot analysis showed that tandutinib did not significantly affect MRP7 expression. Thus, we conclude that the FLT3 inhibitor tandutinib can reverse MRP7-mediated MDR through inhibition of the drug efflux function and may have potential to be used clinically in combination therapy for cancer patients.
Tandutinib (MLN518/CT53518) targeted to stem-like cells by inhibiting the function of ATP-binding cassette subfamily G member 2.[Pubmed:23619284]
Eur J Pharm Sci. 2013 Jun 14;49(3):441-50.
Tandutinib is a novel inhibitor of tyrosine kinases FLT3, PDGFR and KIT. Our study was to explore the capability of tandutinib to reverse ABC transporter-mediated multidrug resistance. Tandutinib reversed ABCG2-mediated drug resistance in ABCG2-482-R2, ABCG2-482-G2, ABCG2-482-T7 and S1-M1-80 cells and increased the accumulation of doxorubicin, rhodamine 123 and [H(3)] mitoxantrone in ABCG2-overexpressing cells. Importantly, tandutinib selectively sensitized side population cells to mitoxantrone. Taken together, our results advocate the potency of tandutinib as an ABCG2 modulator and stem-like cells targeted agent to increase efficiency of anticancer drugs.
The FLT3 inhibitor tandutinib (formerly MLN518) has sequence-independent synergistic effects with cytarabine and daunorubicin.[Pubmed:19625780]
Cell Cycle. 2009 Aug 15;8(16):2621-30.
AML remains a difficult disease to treat. Despite response to induction chemotherapy, most patients ultimately relapse. Further, among elderly patients, aggressive therapy options are often limited due to other medical conditions and decreased tolerance of these patients to conventional chemotherapy. Internal tandem duplications (ITD) of the FLT3 juxtamembrane domain occur in 20-30% of AML patients and predict poor outcome. First clinical data with the FLT3 inhibitor tandutinib demonstrated antileukemic activity in approximately half of the patients--predominantly with FLT3 ITD positive AML. But the data also show that optimal use of tandutinib will require combination therapy with cytotoxic agents. Notably, single agent tandutinib has not been associated with myelosuppression, mucositis or cardiac toxicity--the dose limiting toxicities of AML chemotherapy. We determined the feasibility of combining tandutinib with the standard "3 + 7" induction regimen in AML and show that, in contrast to other structurally unrelated FLT3 inhibitors recently evaluated in clinical trials, the use of tandutinib displayed application sequence independent synergistic antileukemic effects in combination with cytarabine and daunorubicin. Strong synergistic antiproliferative and proapoptotic effects were thereby predominantly seen on FLT3 ITD positive blasts. Further we demonstrate, that addition of tandutinib may allow dose reduction of chemotherapy without loss of overall antileukemic activity--resulting in a potential decrease of side effects. This approach might be an interesting novel strategy especially in the treatment of elderly/comorbid patients. Our data provide a rationale for combining tandutinib with induction chemotherapy in intensified as well as in dose reduction protocols for FLT3 ITD positive AML.
A phase II study of tandutinib (MLN518), a selective inhibitor of type III tyrosine receptor kinases, in patients with metastatic renal cell carcinoma.[Pubmed:20711630]
Invest New Drugs. 2012 Feb;30(1):364-7.
Therapies which target VEGF and mTOR are now available for patients with metastatic renal cell carcinoma, but there is a continued need to develop agents for patients who become refractory to these initial agents. Tandutinib is a relatively selective inhibitor of type III tyrosine kinase receptor kinases with promising activity in some tumors. In this trial, 10 patients with metastatic renal cell carcinoma refractory to previous therapy with sunitinib or sorafenib (median age 61 years, 80% performance status 0, 60% intermediate MSKCC risk classification) received tandutinib 500 mg bid daily with RECIST-defined response as the primary endpoint and progression-free survival (PFS) and overall survival (OS) as secondary endpoints. No patient had more than 2 cycles of therapy and 50% of patients only received 1 cycle with 70% of patients discontinuing for progressive disease and 30% for toxicity. Tandutinib was not well tolerated with dose reduction in 60% of patients due to adverse events. The most common grade 3 toxicity was fatigue (30%). Tandutinib had no clinical activity and due to the excessive toxicity should not be developed further in patients with sunitinib or sorafenib-refractory metastatic renal cell carcinoma.


