DrospirenoneCAS# 67392-87-4 |
- Dofetilide
Catalog No.:BCC3770
CAS No.:115256-11-6
- Repaglinide
Catalog No.:BCC2504
CAS No.:135062-02-1
- NS309
Catalog No.:BCC1809
CAS No.:18711-16-5
- TRAM-34
Catalog No.:BCC1122
CAS No.:289905-88-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

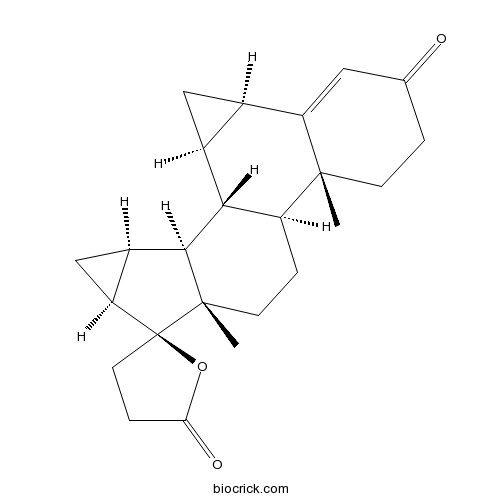
| Cas No. | 67392-87-4 | SDF | Download SDF |
| PubChem ID | 68873 | Appearance | Powder |
| Formula | C24H30O3 | M.Wt | 366.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Dihydrospirorenone | ||
| Solubility | DMSO : 50 mg/mL (136.43 mM; Need ultrasonic) | ||
| SMILES | CC12CCC(=O)C=C1C3CC3C4C2CCC5(C4C6CC6C57CCC(=O)O7)C | ||
| Standard InChIKey | METQSPRSQINEEU-HXCATZOESA-N | ||
| Standard InChI | InChI=1S/C24H30O3/c1-22-6-3-12(25)9-17(22)13-10-14(13)20-16(22)4-7-23(2)21(20)15-11-18(15)24(23)8-5-19(26)27-24/h9,13-16,18,20-21H,3-8,10-11H2,1-2H3/t13-,14+,15-,16+,18+,20-,21+,22-,23+,24+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Drospirenone(Dihydrospirorenone) is a synthetic progestin that is an analog to spironolactone.
Target: Progesterone Receptor
Drospirenone is a novel progestin under clinical development that is similar to the natural hormone progesterone, combining potent progestogenic with antimineralocorticoid and antiandrogenic activities. drospirenone was devoid of glucocorticoid activity. Both progestins did not show any antiglucocorticoid action. Furthermore, drospirenone and progesterone both showed considerable antimineralocorticoid activity and weak mineralocorticoid activity [1]. the pharmacological profile of drospirenone is more closely related to that of the natural hormone progesterone than is that of any other synthetic progestogen in use today. Therefore, drospirenone is anticipated to give rise to a number of additional health benefits both for users of oral contraceptives and hormone replacement therapy recipients [2]. The combination of 17beta-estradiol and drospirenone has a positive effect on BMD and a potentially beneficial effect on lipids. Although endometrial thickness increased slightly, the safety of the endometrium was assured, as no cases of hyperplasia or cancer occurred [3].
Clinical indications: Acne; Dysmenorrhea; Endometriosis; Female contraception; Folic acid deficiency; Premenstrual syndrome References: | |||||

Drospirenone Dilution Calculator

Drospirenone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7286 mL | 13.6429 mL | 27.2859 mL | 54.5717 mL | 68.2147 mL |
| 5 mM | 0.5457 mL | 2.7286 mL | 5.4572 mL | 10.9143 mL | 13.6429 mL |
| 10 mM | 0.2729 mL | 1.3643 mL | 2.7286 mL | 5.4572 mL | 6.8215 mL |
| 50 mM | 0.0546 mL | 0.2729 mL | 0.5457 mL | 1.0914 mL | 1.3643 mL |
| 100 mM | 0.0273 mL | 0.1364 mL | 0.2729 mL | 0.5457 mL | 0.6821 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Drospirenone is a synthetic progestin that is an analog to spironolactone.
- Catalposide
Catalog No.:BCN4225
CAS No.:6736-85-2
- Pterokaurane R
Catalog No.:BCN4076
CAS No.:67349-43-3
- (R,R)-Formoterol
Catalog No.:BCC1293
CAS No.:67346-49-0
- Hexa-D-arginine
Catalog No.:BCC6269
CAS No.:673202-67-0
- 1,3,5-Trihydroxyxanthone
Catalog No.:BCN3357
CAS No.:6732-85-0
- Kaurenoic acid
Catalog No.:BCN4600
CAS No.:6730-83-2
- 2-Hydroxy-4-methoxybenzaldehyde
Catalog No.:BCN7798
CAS No.:673-22-3
- H-Asp(OtBu)-OMe.HCl
Catalog No.:BCC2892
CAS No.:2673-19-0
- H-D-Phe-OH
Catalog No.:BCC3012
CAS No.:673-06-3
- Kansuinine E
Catalog No.:BCN3769
CAS No.:672945-84-5
- 3-O-(2'E ,4'Z-decadienoyl)-20-deoxyingenol
Catalog No.:BCN1382
CAS No.:672941-64-9
- Kif15-IN-2
Catalog No.:BCC5153
CAS No.:672926-33-9
- H-D-Lys-OMe.2HCl
Catalog No.:BCC2680
CAS No.:67396-08-1
- 3-O-Acetyl-11-keto-beta-boswellic acid
Catalog No.:BCN1381
CAS No.:67416-61-9
- Z-D-His-OH
Catalog No.:BCC2767
CAS No.:67424-93-5
- (±)-Blebbistatin
Catalog No.:BCC7169
CAS No.:674289-55-5
- Isorhamnetin-3-O-galactoside
Catalog No.:BCC8190
CAS No.:6743-92-6
- Fmoc-Cys(tBu)-OH
Catalog No.:BCC3478
CAS No.:67436-13-9
- Isoasatone A
Catalog No.:BCN7762
CAS No.:67451-73-4
- Amorolfine
Catalog No.:BCC8819
CAS No.:67467-83-8
- GBR 13069 dihydrochloride
Catalog No.:BCC6640
CAS No.:67469-45-8
- Vanoxerine
Catalog No.:BCC5130
CAS No.:67469-69-6
- GBR 12783 dihydrochloride
Catalog No.:BCC6676
CAS No.:67469-75-4
- Vanoxerine dihydrochloride
Catalog No.:BCC5129
CAS No.:67469-78-7
Drospirenone reduces inflammatory cytokines, vascular endothelial growth factor (VEGF) and nerve growth factor (NGF) expression in human endometriotic stromal cells.[Pubmed:28064076]
J Reprod Immunol. 2017 Feb;119:44-48.
Drospirenone has been used as a progestin in oral contraceptives with ethinyl estradiol (DRSP/EE) and is expected to regulate endometriosis, however, the direct effects of Drospirenone on endometriosis have not been clarified. The aim of this study was to evaluate the anti-inflammatory, anti-angiogenic and anti-neurogenic effects of Drospirenone on endometriotic stromal cells (ESC). ESC isolated from endometriotic tissues were obtained from patients during laparoscopic surgery for ovarian endometriosis. ESC were exposed to IL-1beta and cultured in the absence or presence of Drospirenone. mRNA expression was evaluated using quantitative RT-PCR, and protein was measured using ELISAs. To evaluate the effect of Drospirenone on progesterone receptor (PR) and mineralocorticoid receptor (MR), ESC were transfected with siRNA against PR (siPR) and MR (siMR), and cultured in the presence or absence of Drospirenone. Drospirenone significantly decreased IL-6, IL-8, VEGF and NGF mRNA expression by ESC. Drospirenone (10(-5)M) significantly decreased IL-6 secretion and 10(-7)M Drospirenone decreased IL-8 and VEGF secretion. Knockdown of PR, but not MR, negated the effects of Drospirenone. In summary, this study demonstrates that Drospirenone has anti-inflammatory, anti-angiogenic and anti-neurogenic effects on ESC and these effects are mediated by PR. These Drospirenone effects may contribute to the regulatory effects of Drospirenone-containing oral contraceptives on endometriosis.
Efficacy and safety of drospirenone 2 mg/17beta-estradiol 1 mg hormone therapy in Korean postmenopausal women.[Pubmed:28344964]
Obstet Gynecol Sci. 2017 Mar;60(2):213-217.
This regulatory post-marketing surveillance study aimed to evaluate the therapeutic efficacy and safety of Drospirenone (DRSP) 2 mg/estradiol (E2) 1 mg tablet in Korean postmenopausal women. A total of 4,149 patients were enrolled and the study was conducted at 207 clinical research centers. The patients' source data was collected between November 2006 and November 2012. More than 85% of patients experienced improvement of menopausal symptoms. The most frequently reported adverse events were vaginal bleeding and breast pain; most of the women suffering from these symptoms fully recovered. The incidence of adverse event was higher in patients of younger age (20 to 39 years), in patients with concomitant diseases, previous hormone replacement therapy in medical history, those treated with DRSP 2 mg/E2 1 mg for shorter duration (3 years or less) and in patients using concomitant medication. In conclusion, the results from this large post-marketing surveillance study confirm the efficacy and safety of DRSP 2 mg/E2 1 mg tablet in Korean postmenopausal women.
Drospirenone-containing oral contraceptive pills and the risk of venous thromboembolism: a systematic review of observational studies.[Pubmed:28276140]
BJOG. 2017 Sep;124(10):1490-1499.
BACKGROUND: The effects of fourth-generation Drospirenone-containing combined oral contraceptives (COCs) on the risk of venous thromboembolism (VTE) are controversial. OBJECTIVES: To assess the methodological strengths and limitations of the evidence on the VTE risk of these COCs. SEARCH STRATEGY: We searched CINAHL, the Cochrane Library, EMBASE, HealthStar, Medline, and the Science Citation Index. SELECTION CRITERIA: Studies were included if they were cohort and case-control studies, reported a venous thrombotic outcome, had a comparator group, reported an effect measure of the association of interest, and were published in English or French. DATA COLLECTION AND ANALYSIS: We assessed study quality using the ROBINS-I tool and assessed the presence of four common sources of bias: prevalent user bias, inappropriate choice of comparator, VTE misclassification, and confounding. MAIN RESULTS: Our systematic review included 17 studies. The relative risks of VTE associated with Drospirenone- versus second-generation levonorgestrel-containing COCs ranged from 1.0 to 3.3. Based on ROBINS-I, three studies had a moderate risk, ten had a serious risk, and four had a critical risk. Nine studies included prevalent users, four included inappropriate comparators, four had VTE misclassification, and five did not account for two or more important confounding factors. The three highest quality studies had relative risks ranging from 1.0 to 1.57. AUTHOR'S CONCLUSIONS: As a result of the methodological limitations of the individual studies, the VTE risk of Drospirenone-containing COCs remains unknown. The highest quality studies suggest there are no or slightly increased harmful effects, but their confidence limits do not rule out an almost doubling of the risk. TWEETABLE ABSTRACT: Systematic review of Drospirenone: best studies show no or slightly increased VTE risk (versus levonorgestrel).
Pooled analysis of two randomized, open-label studies comparing the effects of nomegestrol acetate/17beta-estradiol and drospirenone/ethinyl estradiol on bleeding patterns in healthy women.[Pubmed:28011288]
Contraception. 2017 Apr;95(4):390-397.
OBJECTIVES: To obtain more precise and detailed information regarding the bleeding patterns of nomegestrol acetate (NOMAC)/17beta-estradiol (E2) and Drospirenone/ethinyl estradiol (DRSP/EE) and to identify whether baseline demographic characteristics were associated with unscheduled bleeding, absent scheduled bleeding, or amenorrhea. STUDY DESIGN: This analysis pooled results from two pivotal open-label, randomized trials that compared bleeding patterns of NOMAC/E2 and DRSP/EE. In the two studies 4317 women aged 18-50 years from 24 countries across the Americas, Europe, and Asia underwent treatment. RESULTS: 2835 women taking NOMAC/E2 (2.5 mg/1.5 mg) in a 24/4-day regimen and 938 women taking DRSP/EE (3 mg/30 mug) in a 21/7-day regimen had at least 1 evaluable cycle for vaginal bleeding analyses. The frequency of absent scheduled bleeding was higher (p<.0001) for women using NOMAC/E2 than DRSP/EE across all 11 cycles (cycles 2-12), ranging between 17.6% and 31.6% and between 3.4% and 5.8%, respectively. For women who had absent scheduled bleeding in cycles 2, 3, or 4 the incidence of absent scheduled bleeding in subsequent cycles was high and ranged between approximately 50%-60% for NOMAC/E2 and approximately 40%-50% for DRSP/EE. Amenorrhea increased over time with both regimens, being higher with NOMAC/E2. Both absent scheduled bleeding and amenorrhea with NOMAC/E2 were more common in older women, overweight women, switchers, and smokers; unscheduled bleeding was more common in starters, but had no association with age, body mass index, and smoking. CONCLUSIONS: NOMAC/E2 is associated with a higher prevalence of absent scheduled bleeding compared with DRSP/EE. Absent scheduled bleeding and amenorrhea were associated with age, body weight, switching and smoking. Unscheduled bleeding was more common in starters. IMPLICATIONS: Information about the factors associated with bleeding patterns may help clinicians provide guidance to women considering use of the NOMAC/E2 oral contraceptive.


