CatalposideCAS# 6736-85-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6736-85-2 | SDF | Download SDF |
| PubChem ID | 93039 | Appearance | Powder |
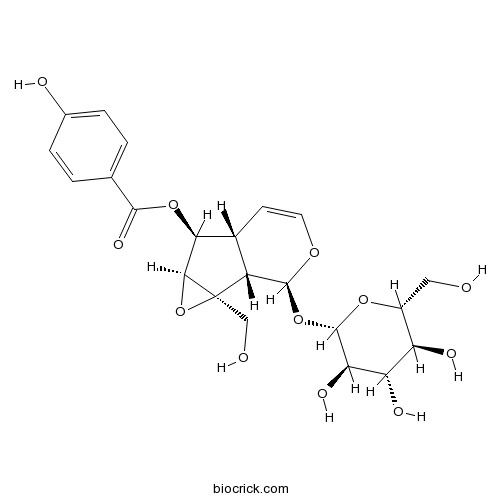
| Formula | C22H26O12 | M.Wt | 482.4 |
| Type of Compound | Iridoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(1aS,1bS,2S,5aR,6S,6aS)-1a-(hydroxymethyl)-2-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-2,5a,6,6a-tetrahydro-1bH-oxireno[5,6]cyclopenta[1,3-c]pyran-6-yl] 4-hydroxybenzoate | ||
| SMILES | C1=COC(C2C1C(C3C2(O3)CO)OC(=O)C4=CC=C(C=C4)O)OC5C(C(C(C(O5)CO)O)O)O | ||
| Standard InChIKey | UXSACQOOWZMGSE-RWORTQBESA-N | ||
| Standard InChI | InChI=1S/C22H26O12/c23-7-12-14(26)15(27)16(28)21(31-12)33-20-13-11(5-6-30-20)17(18-22(13,8-24)34-18)32-19(29)9-1-3-10(25)4-2-9/h1-6,11-18,20-21,23-28H,7-8H2/t11-,12-,13-,14-,15+,16-,17+,18+,20+,21+,22-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Catalposide possesses antioxidant, anti-apoptotic, anti-microbial, anti-tumoral, and anti-inflammatory properties. 2. Catalposide is a potent inducer of HO-1 and HO-1 induction is responsible for the catalposide-mediated cytoprotection against oxidative damage. 3. Catalposide is a natural agonistic ligand of peroxisome proliferator-activated receptor-α, is hypolipidemic by activation of PPARαvia a ligand-mediated mechanism that modulates the expression of in lipid metabolism genes in hepatocytes. 4. Catalposide can attenuate induction of intestinal epithelial proinflammatory gene expression and reduce the severity of trinitrobenzene sulfonic acid-induced colitis in mice, it may be an effective agent for the treatment of diseases characterized by mucosal inflammation. |
| Targets | PPAR | NO | NOS | HO-1 | NF-kB | p65 | TNF-α | IL Receptor | ERK |

Catalposide Dilution Calculator

Catalposide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.073 mL | 10.3648 mL | 20.7297 mL | 41.4594 mL | 51.8242 mL |
| 5 mM | 0.4146 mL | 2.073 mL | 4.1459 mL | 8.2919 mL | 10.3648 mL |
| 10 mM | 0.2073 mL | 1.0365 mL | 2.073 mL | 4.1459 mL | 5.1824 mL |
| 50 mM | 0.0415 mL | 0.2073 mL | 0.4146 mL | 0.8292 mL | 1.0365 mL |
| 100 mM | 0.0207 mL | 0.1036 mL | 0.2073 mL | 0.4146 mL | 0.5182 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Pterokaurane R
Catalog No.:BCN4076
CAS No.:67349-43-3
- (R,R)-Formoterol
Catalog No.:BCC1293
CAS No.:67346-49-0
- Hexa-D-arginine
Catalog No.:BCC6269
CAS No.:673202-67-0
- 1,3,5-Trihydroxyxanthone
Catalog No.:BCN3357
CAS No.:6732-85-0
- Kaurenoic acid
Catalog No.:BCN4600
CAS No.:6730-83-2
- 2-Hydroxy-4-methoxybenzaldehyde
Catalog No.:BCN7798
CAS No.:673-22-3
- H-Asp(OtBu)-OMe.HCl
Catalog No.:BCC2892
CAS No.:2673-19-0
- H-D-Phe-OH
Catalog No.:BCC3012
CAS No.:673-06-3
- Kansuinine E
Catalog No.:BCN3769
CAS No.:672945-84-5
- 3-O-(2'E ,4'Z-decadienoyl)-20-deoxyingenol
Catalog No.:BCN1382
CAS No.:672941-64-9
- Kif15-IN-2
Catalog No.:BCC5153
CAS No.:672926-33-9
- Kif15-IN-1
Catalog No.:BCC5152
CAS No.:672926-32-8
- Drospirenone
Catalog No.:BCC4493
CAS No.:67392-87-4
- H-D-Lys-OMe.2HCl
Catalog No.:BCC2680
CAS No.:67396-08-1
- 3-O-Acetyl-11-keto-beta-boswellic acid
Catalog No.:BCN1381
CAS No.:67416-61-9
- Z-D-His-OH
Catalog No.:BCC2767
CAS No.:67424-93-5
- (±)-Blebbistatin
Catalog No.:BCC7169
CAS No.:674289-55-5
- Isorhamnetin-3-O-galactoside
Catalog No.:BCC8190
CAS No.:6743-92-6
- Fmoc-Cys(tBu)-OH
Catalog No.:BCC3478
CAS No.:67436-13-9
- Isoasatone A
Catalog No.:BCN7762
CAS No.:67451-73-4
- Amorolfine
Catalog No.:BCC8819
CAS No.:67467-83-8
- GBR 13069 dihydrochloride
Catalog No.:BCC6640
CAS No.:67469-45-8
- Vanoxerine
Catalog No.:BCC5130
CAS No.:67469-69-6
- GBR 12783 dihydrochloride
Catalog No.:BCC6676
CAS No.:67469-75-4
Electrochemical and spectrometric study of antioxidant activity of pomiferin, isopomiferin, osajin and catalposide.[Pubmed:18597965]
J Pharm Biomed Anal. 2008 Sep 10;48(1):127-33.
The antioxidant properties of pomiferin, isopomiferin, osajin and Catalposide are evaluated. The electrochemical behaviour of these compounds at a carbon paste electrode was studied using square wave voltammetry. Oxidative signals, optimized frequencies and appropriate pH acetate buffer conditions were determined. The detection limits (3S/N) for pomiferin, isopomiferin, osajin and Catalposide were estimated to be 50 pg/ml, 800 pg/ml, 40 pg/ml and 10 ng/ml, respectively. Furthermore, spectrometric test was employed with 2,2-diphenyl-1-picrylhydrazyle (DPPH) to evaluate the antioxidant activities of these compounds. Based on the obtained results, the highest antioxidant activity measured by DPPH tests was found at pomiferin followed by isopomiferin. The activities of osajin and Catalposide were undetectable. The protective effects of pomiferin, isopomiferin, osajin and Catalposide on DNA exposed to oxygen radicals in vitro were also studied. Changes in height of oxidative signals for the four bases (guanine, thymine, adenine and cytosine) were measured for DNA exposed to oxygen radicals, generated by Fenton's reaction, non-oxidized ssDNA (50 microg/ml) displayed well developed signals; however, after oxidative damage the observed oxidative signals decreased. Significant protective effects were observed for pomiferin and osajin. Decreased effect was observed for isopomiferin while a further reduced protective effect was seen for DNA exposed to Catalposide. Based on the obtained results, pomiferin had the highest antioxidant activity followed by isopomiferin, osajin and Catalposide.
Inhibition of inducible nitric oxide synthesis by catalposide from Catalpa ovata.[Pubmed:12221588]
Planta Med. 2002 Aug;68(8):685-9.
Catalposide (1) and two related iridoids were isolated from the stem of Catalpa ovata (Bignoniaceae) by bioassay guided fractionation. Catalposide (1) significantly inhibited the production of nitric oxide (NO) in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages in a dose-dependent manner. RT-PCR and Western blot analyses demonstrated that Catalposide (1) suppressed the expression of inducible nitric oxide synthase (iNOS) gene and iNOS protein. Catalposide (1) also inhibited the activation of LPS-induced NF-kappaB as analyzed by electrophoretic mobility shift assay (EMSA). In addition to the inhibitory effect on NO production in LPS-stimulated RAW 264.7 cells, Catalposide (1) significantly inhibited the NO production in cytokine-stimulated human DLD-1 and rat vascular smooth muscle (VSM) cells in a dose-dependent manner.
Catalposide, a compound isolated from catalpa ovata, attenuates induction of intestinal epithelial proinflammatory gene expression and reduces the severity of trinitrobenzene sulfonic Acid-induced colitis in mice.[Pubmed:15472516]
Inflamm Bowel Dis. 2004 Sep;10(5):564-72.
Certain irinoid-producing plants have been used as herbal anti-inflammatory remedies. Here we evaluated whether Catalposide (CATP), a single compound isolated from irinoid-producing plant Catalpa ovata, has a potential for preventing or ameliorating diseases characterized by mucosal inflammation. Preliminary microarray-based gene expression test revealed that CATP, which alone did not significantly affect expression of any of the >8,000 genes analyzed, attenuated the expression of tumor necrosis factor-alpha (TNF-alpha)-induced proinflammatory genes including interleukin-8 (IL-8) in human intestinal epithelial HT-29 cells. Down-regulation of IL-8 mRNA accumulation was also reflected by the decreased IL-8 secretion in CATP-treated HT-29 cells. The signal transduction study revealed that CATP significantly attenuates TNF-alpha-mediated p38 and extracellular signal-regulated kinase (ERK) phosphorylation. Further, CATP reduced NF-kappaB-mediated transcriptional activation as well as Ikappa-Balpha degradation. To establish the in vivo relevance of these findings, we examined whether CATP could affect intestinal inflammation in vivo using the mouse model of trinitrobenzene sulfonic acid (TNBS)-induced inflammatory colitis. Intrarectal administration of CATP dramatically reduced the weight loss, colonic damage, and mucosal ulceration that characterize TNBS colitis. Moreover, CATP suppressed the expression of TNF-alpha, interleukin-1beta, and intercellular adhesion molecule-1 along with the inhibition of NF-kappa B p65 translocation into nucleus in TNBS colitis. Collectively, current results demonstrate that CATP may be an effective agent for the treatment of diseases characterized by mucosal inflammation.
Catalposide is a natural agonistic ligand of peroxisome proliferator-activated receptor-alpha.[Pubmed:22583896]
Biochem Biophys Res Commun. 2012 Jun 15;422(4):568-72.
Peroxisome proliferator-activated receptor-alpha (PPARalpha) is a nuclear receptor that regulates the expression of genes related to cellular lipid uptake and oxidation. Thus, PPARalpha agonists may be important in the treatment of hypertriglyceridemia and hepatic steatosis. In this study, we demonstrated that Catalposide is a novel natural PPARalpha agonist, identified from reporter gene assay-based activity screening with approximately 900 natural plant and seaweed extracts. Results of time-resolved fluorescence resonance energy transfer analyses suggested that the compound interacted directly with the ligand-binding domain of PPARalpha. Cultured hepatocytes stimulated with Catalposide exhibited significantly reduced cellular triglyceride concentrations, by 21%, while cellular uptake of fatty acids was increased, by 70% (P<0.05). Quantitative PCR analysis revealed that the increase in cellular fatty acid uptake was due to upregulation of fatty acid transporter protein-4 (+19% vs. the control) in cells stimulated with Catalposide. Additionally, expression of genes related to fatty acid oxidation and high-density lipoprotein metabolism were upregulated, while that of genes related to fatty acid synthesis were suppressed. In conclusion, Catalposide is hypolipidemic by activation of PPARalpha via a ligand-mediated mechanism that modulates the expression of in lipid metabolism genes in hepatocytes.
Inhibition of TNF-alpha, IL-1beta, and IL-6 productions and NF-kappa B activation in lipopolysaccharide-activated RAW 264.7 macrophages by catalposide, an iridoid glycoside isolated from Catalpa ovata G. Don (Bignoniaceae).[Pubmed:12349954]
Int Immunopharmacol. 2002 Jul;2(8):1173-81.
Catalposide, the major iridoid glycoside isolated from the stem bark of Catalpa ovata G. Don (Bignoniaceae), was found to inhibit the productions of tumor necrosis factor-alpha (TNF-alpha), interleukin-1beta (IL-1beta), and interleukin-6 (IL-6), and the activation of nuclear factor kappaB (NF-kappaB) in RAW 264.7 macrophages activated with lipopolysaccharide (LPS). Catalposide also inhibited the expressions of TNF-alpha, IL-1beta, and IL-6 genes and the nuclear translocation of p65 subunit of NF-kappaB in LPS-activated RAW 264.7 cells. Flow cytometric analysis revealed that Catalposide suppressed the binding of FITC-conjugated LPS to CD14 on the surface of cells, probably resulting in the inhibitory effects on TNF-alpha, IL-1beta, and IL-6 productions and NF-kappaB activation. These findings suggest that Catalposide could be an attractive candidate for adjunctive therapy in gram-negative bacterial infections.
Catalposide protects Neuro 2A cells from hydrogen peroxide-induced cytotoxicity via the expression of heme oxygenase-1.[Pubmed:12962973]
Toxicol Lett. 2003 Nov 1;145(1):46-54.
Catalposide, the major iridoid glycoside isolated from the stem bark of Catalpa ovata G. Don (Bignoniaceae) has been shown to possess anti-microbial, anti-tumoral, and anti-inflammatory properties. Heme oxygenase-1 (HO-1) is a stress response protein and is known to play a protective role against the oxidative injury. In this study, we examined whether Catalposide could protect Neuro 2A cells, a kind of neuronal cell lines, from oxidative damage through the induction of HO-1 protein expression and HO activity. The treatment of the cells with Catalposide resulted in dose- and time-dependent up-regulations of both HO-1 protein expression and HO activity. Catalposide protected the cells from hydrogen peroxide-induced cell death. The protective effect of Catalposide on hydrogen peroxide-induced cell death was abrogated by zinc protoporphyrin IX (ZnPP IX), a HO inhibitor. Additional experiments revealed the involvement of CO in the cytoprotective effect of Catalposide-induced HO-1. These results indicate that Catalposide is a potent inducer of HO-1 and HO-1 induction is responsible for the Catalposide-mediated cytoprotection against oxidative damage.


